Adeno-associated virus expressing a blood-brain barrier-penetrating enzyme improves GM1 gangliosidosis in a preclinical model
- PMID: 40198143
- PMCID: PMC12165788
- DOI: 10.1172/JCI180724
Adeno-associated virus expressing a blood-brain barrier-penetrating enzyme improves GM1 gangliosidosis in a preclinical model
Abstract
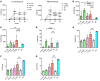
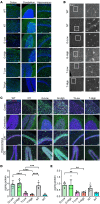
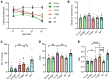
GM1 gangliosidosis is a lysosomal storage disorder (LSD) caused by genetic defects in lysosomal β-galactosidase (β-gal). The primary substrate of β-gal is GM1 ganglioside (GM1), a sialylated glycosphingolipid abundant in the central nervous system (CNS). Deficiency in β-gal causes GM1 to accumulate in neural cells, leading to a rapid decline in psychomotor functions, seizures, and premature death. There is currently no therapy available. Although enzyme replacement therapy has been approved for other LSDs, its effects on the CNS are limited owing to the blood-brain barrier (BBB). Here, we assessed the therapeutic efficacy of a systemic infusion of an adeno-associated virus vector carrying a gene expressing a BBB-penetrable enzyme under the control of a liver-specific promoter in GM1 gangliosidosis model mice. The BBB-penetrable enzyme consisted of the variable region of the anti-transferrin receptor antibody fused with β-gal. The BBB-penetrable enzyme was only produced in the liver and secreted into the blood, which was efficiently distributed to various organs, including the brain. GM1 accumulation in the CNS was completely normalized, with improved neurological functions and animal survival. This therapeutic approach is expected to be applied for the treatment of several hereditary neurological diseases with CNS involvement.
Keywords: Gene therapy; Genetics; Lysosomes; Therapeutics.
Conflict of interest statement
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

