Epigenetics and Mitochondrial Biogenesis: The Role of Sirtuins in HIV Neuropathogenesis
- PMID: 40198445
- PMCID: PMC12289736
- DOI: 10.1007/s12035-025-04885-7
Epigenetics and Mitochondrial Biogenesis: The Role of Sirtuins in HIV Neuropathogenesis
Abstract
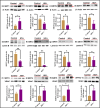
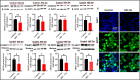
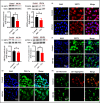
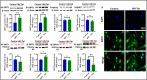
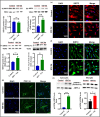
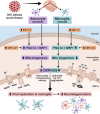
Mitochondrial energy deficits play a central role in HIV-associated neurocognitive disorder (HAND). HIV disrupts cellular functions, including epigenetic modifications such as class III histone deacetylation mediated by sirtuins (SIRTs). However, the role of SIRTs in HAND pathogenesis remains unclear. We hypothesize that HIV alters mitochondrial biogenesis and energy homeostasis by modifying SIRT family members 1-7, contributing to HAND progression. To test this hypothesis, we examined postmortem frontal lobe brain tissue from people with HIV (PWH) and HIV-negative controls, focusing on epigenetic alterations in SIRTs 1-7, the energy sensor adenosine monophosphate-activated protein kinase (AMPK), the mitochondrial master regulator peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), and transcription factors such as mitochondrial transcription factor A (TFAM), nuclear respiratory factors 1 and 2 (NRF-1/2), and factors associated with oxidative phosphorylation (OXPHOS). Our analysis revealed a significant increase in AMPK, OXPHOS, and PGC-1α levels, alongside a decrease in TFAM levels in PWH brains compared to uninfected controls. NRF-1 was upregulated in mitochondria but downregulated in the cytoplasm, while NRF-2 exhibited the opposite trend in PWH compared to HIV-negative controls. The epigenetic signatures of SIRTs 1, 2, 3, 4, 6, and 7 were upregulated in PWH, while SIRT5 was downregulated compared to uninfected brain tissues. We exposed primary human astrocyte and microglial cultures to the HIV-1 transactivator of transcription (Tat) protein to identify the cell types involved. These studies confirmed that HIV-induced epigenetic modifications of SIRTs and mitochondrial impairments occurred in both astrocytes and microglia, highlighting the crucial role of SIRTs in HAND pathogenesis.
Keywords: Epigenetic modification; HAND; HIV; Mitochondrial biogenesis; Sirtuins.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Institutional Review Board Statement: Brain tissue samples from uninfected and HIV-positive frontal and temporal regions were obtained from the National NeuroAIDS Tissue Consortium (NNTC) and National Neurological AIDS Bank (NNAB) University of California (UCLA), California, USA. Informed Consent: Not applicable. Competing Interest: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

