In danger: HIV vaccine research and development in Europe
- PMID: 40198605
- PMCID: PMC11977976
- DOI: 10.1371/journal.pgph.0004364
In danger: HIV vaccine research and development in Europe
Abstract
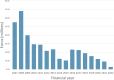
Highly effective antiretroviral-based HIV prevention plays an important role in ending the global HIV/AIDS epidemic. However, the sustainable control of the epidemic is hampered by unequal access to prevention options, including HIV testing, alongside with drug resistance and ongoing barriers to accessing sustainable HIV treatment. Therefore, an HIV vaccine, combined with effective prevention and treatment, remains an absolute necessity to control the epidemic. Yet, the recent discontinuation of four major vaccine efficacy studies is raising concerns about the future of HIV vaccine research and development globally, and particularly in the European region where funding for vaccine research and development has shrinked. This viewpoint emphasises that supporting HIV vaccine research and development at the European level remains crucial: it is not only necessary to control the epidemic, but it promotes innovation, strengthens health security, epidemic preparedness, and health sovereignty while contributing to the economies of European nations.
Copyright: © 2025 Tatoud et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Roger Tatoud declares that he is a consultant for the IAS. Christan Brander is a named inventor in patents WO/2013/110818 and US 61/412060. Behazine Combadière reports a relationship with Sanofi Pasteur Inc. that includes employment and equity or stocks. Tomáš Hanke is a named inventor on the HIVconsvX immunogen patent protected under EP14846993.5 and PCT/US14/58422 (WO2015048785). Gunilla Karlsson Hedestam reports being unpaid scientific advisory board member for HIV Vaccine Trials Network (HVTN). Rogier W. Sanders has patent licensed to International AIDS Vaccine Initiative, The Scripps Research Institute, Cornell University (US patent application 16/642,131 and EP2765138B1). He also reports funding from Janssen. Alexandra Trkola reports funding from the Gilead Foundation, Novartis Foundation and being on advisory boards for the German Centre for Infections Research (DZIF), Institute for Research in Biomedicine (IRB), Scripps Consortium for HIV/AIDS Vaccine Development (CHAVD) and Centre for the AIDS Programme of Research in South Africa (CAPRISA). Marit J. Van Gills reports being board member of Netherlands Conference on HIV Pathogenesis, Epidemiology, Prevention and Treatment (NCHIV). Authors receive funding from a range of not-for-profit organisations, which had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. None of these competing interests in any way alter our adherence to PLOS Global Public Health policies on sharing data and materials.
Figures
References
-
- Gokengin D, Bursa D, Skrzat-Klapaczynska A, Alexiev I, Arsikj E, Balayan T, et al.. PrEP Scale-Up and PEP in central and eastern Europe: changes in time and the challenges we face with no expected HIV vaccine in the near future. Vaccines (Basel). 2023;11(1):122. doi: 10.3390/vaccines11010122 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources