HOPS/CORVET tethering complexes are critical for endocytosis and protein trafficking to invasion related organelles in malaria parasites
- PMID: 40198740
- PMCID: PMC12011295
- DOI: 10.1371/journal.ppat.1013053
HOPS/CORVET tethering complexes are critical for endocytosis and protein trafficking to invasion related organelles in malaria parasites
Abstract
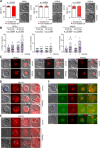
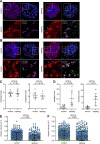
The tethering complexes HOPS/CORVET are central for vesicular fusion through the eukaryotic endolysosomal system, but the functions of these complexes in the intracellular development of malaria parasites are still unknown. Here we show that the HOPS/CORVET core subunits are critical for the intracellular proliferation of the malaria parasite Plasmodium falciparum. We demonstrate that HOPS/CORVET are required for parasite endocytosis and host cell cytosol uptake, as early functional depletion of the complex led to developmental arrest and accumulation of endosomes that failed to fuse to the digestive vacuole membrane. Late depletion of the core HOPS/CORVET subunits led to a severe defect in merozoite invasion as a result of the mistargeting of proteins destined to the apical secretory organelles, the rhoptries and micronemes. Ultrastructure-expansion microscopy revealed a reduced rhoptry volume and the accumulation of numerous vesicles in HOPS/CORVET deficient schizonts, further supporting a role of HOPS/CORVET in post-Golgi protein cargo trafficking to the invasion related organelles. Hence, malaria parasites have repurposed HOPS/CORVET to perform dual functions across the intraerythrocytic cycle, consistent with a canonical endocytic pathway for delivery of host cell material to the digestive vacuole in trophozoite stages and a parasite specific role in trafficking of protein cargo to the apical organelles required for invasion in schizont stages.
Copyright: © 2025 Mesén-Ramírez et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- WHO. World malaria report 2023. 2023. Report No.: ISBN 978-92-4-008617-3.
-
- Poespoprodjo J, Douglas N, Ansong D, Kho S, Anstey N. Malaria. Lancet. 2023;402(10419):2328–45. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

