Letter to the FDA Proposing Major Changes in the US Clozapine Package Insert Supported by Clozapine Experts Worldwide. Part II: A Review of Fatal Outcomes in US Pharmacovigilance Data and Proposed Changes
- PMID: 40198784
- PMCID: PMC12105975
- DOI: 10.1097/JCP.0000000000001990
Letter to the FDA Proposing Major Changes in the US Clozapine Package Insert Supported by Clozapine Experts Worldwide. Part II: A Review of Fatal Outcomes in US Pharmacovigilance Data and Proposed Changes
Abstract
Purpose/background: This is the second part of a 2-part article that proposes improving the United States (US) clozapine package insert. Part II focuses on fatal outcomes and the 5 boxed warnings, 4 specifically for clozapine: severe neutropenia, seizure, orthostatic hypotension and myocarditis, and 1 for all antipsychotics (elderly with dementia).
Methods: US reports to the World Health Organization's global pharmacovigilance database were analyzed from clozapine's introduction to January 15, 2023.
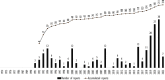
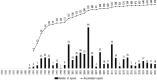
Findings/results: The US was the top reporter worldwide for clozapine with 56,003 reports and 9587 associated fatal outcomes. The 4 clozapine boxed warnings were associated with 534 fatal outcomes (218 with severe neutropenia, 131 with seizures, 125 with orthostasis, 36 with myocarditis, 24 with cardiomyopathy, and 0 with mitral valve prolapse). With no boxed warnings, pneumonia was associated with 674 fatal outcomes and increased white blood cell count (a sign of infection) with 596 fatal outcomes. After considering overlaps, pneumonia and increases in white blood cell count explained 900 fatalities, or 9.4% of 9587 fatal outcomes. The Food and Drug Administration continues to focus on severe neutropenia which was associated with only 218 or 2.3% of fatal outcomes, whereas 97.7% of fatal outcomes reported in US clozapine-treated patients had another cause.
Implications/conclusions: To help prevent future deaths in clozapine-treated patients, the clozapine package insert should focus on fatal outcomes during infections. Part II offers detailed solutions regarding current boxed warnings and lack of a warning for pneumonia and other infections. The Supplementary Material includes letters of support from 124 non-US clozapine experts from 44 countries/regions who support Parts I and II.
Keywords: clozapine/adverse effects; clozapine/therapeutic use; clozapine/toxicity; drug labeling; schizophrenia.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Figures
References
-
- Shader RI. Some thoughts on pharmacovigilance and postmarketing surveillance. Clin Ther. 2016;38:2509–2510. - PubMed
-
- World Health Organization . The Importance of Pharmacovigilance. Safety Monitoring of Medicinal Products. Geneva: WHO; 2002.
-
- Issa AM Phillips KA Van Bebber S, et al. . Drug withdrawals in the United States: a systematic review of the evidence and analysis of trends. Curr Drug Saf. 2007;2:177–185. - PubMed
-
- US Department of Health and Human Services . Guidance for industry warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products -content and format. Published in 2011. Available at: https://www.fda.gov/files/drugs/published/Warnings-and-Precautions--Cont... Accessed August 10, 2024.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

