Long-Term Performance of Two Systems for Automated Insulin Delivery in Adults With Type 1 Diabetes: An Observational Study
- PMID: 40198839
- PMCID: PMC11977919
- DOI: 10.1002/edm2.70043
Long-Term Performance of Two Systems for Automated Insulin Delivery in Adults With Type 1 Diabetes: An Observational Study
Abstract
Aims: To compare glycaemic outcomes for two automated insulin delivery (AID) systems, the Tandem Control IQ (CIQ) and the MiniMed 780G (MM780G).
Material and methods: In this observational study, we evaluated 60 days of glycaemic data from 139 persons with type 1 diabetes (CIQ: 79 persons, MM780G: 60 persons), who had an active glucose sensor time ≥ 85%.
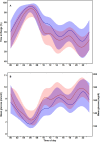
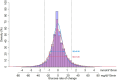
Results: The time with AID was median 620 (IQR, 439-755) days for CIQ users and 509 (429-744) days for MM780G users (p = 0.26). The last HbA1c before initiation of AID was 59.7 mmol/mol in CIQ and 60.1 mmol/mol in MM780G (p = 0.88). The time with an active glucose sensor was higher for CIQ than MM780G (median 98.5 (97.4-98.0)% vs. 96.5 (94.9-97.0)%, p < 0.001). Time in range (TIR, glucose 3.9-10.0 mmol/L) was lower in CIQ than MM780G (mean 68.9% ± 11.4% vs. 73.7% ± 12.0%, p = 0.02) as was time in tight range (TITR) (glucose 3.9-7.8 mmol/L) (43.0% ± 12.2% vs. 48.4% ± 12.7%, p = 0.01). The difference in TIR (4.2 (95% CI 1.0-7.5)%, p = 0.01) and TITR (5.0 (1.4-8.6)%, p < 0.01) remained statistically significant in a multiple regression model controlling for various baseline variables. Time with an absolute rate of glucose change > 1.5 mmol/L/15 min was higher in CIQ than MM780G (9.4 (IQR, 7.2-13.3)% vs. 7.4 (5.2-10.4)%, p < 0.001).
Conclusions: The CIQ system had a higher active glucose sensor time but a lower TIR, TITR, and a higher time with a rapid glucose rate of change than the MM780G system.
Keywords: automated insulin delivery; continuous glucose monitoring; glucose rate of change; glucose time in range; type 1 diabetes.
© 2025 The Author(s). Endocrinology, Diabetes & Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
K.W.H. has received a grant from Abbott Diabetes Care and Novo Nordisk for an investigator‐initiated study.
Figures
References
-
- Limbert C., Kowalski A. J., and Danne T. P. A., “Automated Insulin Delivery: A Milestone on the Road to Insulin Independence in Type 1 Diabetes,” Diabetes Care 47, no. 6 (2024): 918–920. - PubMed
-
- Sherr J. L., Heinemann L., Fleming G. A., et al., “Automated Insulin Delivery: Benefits, Challenges, and Recommendations. A Consensus Report of the Joint Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association,” Diabetes Care 45, no. 12 (2022): 3058–3074. - PubMed
-
- Griffin T. P., Gallen G., Hartnell S., et al., “UK's Association of British Clinical Diabetologist's Diabetes Technology Network (ABCD‐DTN): Best Practice Guide for Hybrid Closed‐Loop Therapy,” Diabetic Medicine 40, no. 7 (2023): e15078. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

