One-Year Radiologic Progression in Sporadic and Hereditary Cerebral Amyloid Angiopathy
- PMID: 40198864
- PMCID: PMC11995281
- DOI: 10.1212/WNL.0000000000213546
One-Year Radiologic Progression in Sporadic and Hereditary Cerebral Amyloid Angiopathy
Abstract
Background and objectives: Knowledge on the short-term progression of cerebral amyloid angiopathy (CAA) is important for clinical practice and the design of clinical treatment trials. We investigated the 1-year progression of CAA-related MRI markers in sporadic (sCAA) and Dutch-type hereditary (D-CAA).
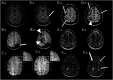
Methods: Participants were included from 2 prospective cohort studies. 3T-MRI was performed at baseline and after 1 year. We assessed macrobleeds, cerebral microbleeds (CMBs), cortical superficial siderosis (cSS), convexity subarachnoid hemorrhages (cSAHs), white matter hyperintensities (WMH), enlarged centrum semiovale perivascular spaces (CSO-EPVS), and visually stimulated blood oxygenation level-dependent (BOLD) fMRI parameters. Progression was defined as increase in number of macrobleeds or CMBs, new focus or extension of cSS, increase in CSO-EPVS category, or volume increase of >10% of WMH. Multivariable regression analyses were performed to determine factors associated with progression and the association between events related to parenchymal injury (cSAH, macrobleeds) and radiologic progression.
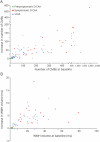
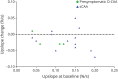
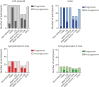
Results: We included 98 participants (47% women): 55 with sCAA (mean age 70 years), 28 with symptomatic D-CAA (mean age 59 years), and 15 with presymptomatic D-CAA (mean age 45 years). Progression of >1 MRI markers was seen in all 83 (100%) participants with sCAA and symptomatic D-CAA and in 9 (60%) with presymptomatic D-CAA. The number of CMBs showed the largest progression in sCAA (98%; median increase 24) and symptomatic D-CAA (100%; median increase 58). WMH volume (>10% increase in 70%; mean increase 1.2 mL) was most progressive in presymptomatic D-CAA. A decrease in the upslope of the visually evoked BOLD response was observed for most patients. Symptomatic D-CAA status was associated with more overall progression (adjusted odds ratio [aOR] 9.7; 95% CI 1.7-54.2), CMB (adjusted relative risk [aRR] 2.47; 95% CI 1.5-4.1), and WMH volume progression (β 2.52; 95% CI 0.3-4.8). Baseline CMB count (aRR 1.002; 95% CI 1.001-1.002) was associated with CMB progression and cSS presence at baseline (aOR 8.16; 95% CI 2.6-25.4) with cSS progression. cSS progression was also associated with cSAH and macrobleeds (aOR 21,029; 95% CI 2.042-216.537).
Discussion: CAA is a radiologically progressive disease even in the short-term. After 1 year, all symptomatic and most of the presymptomatic participants showed progression of at least 1 MRI-marker. CMBs and WMH volume (in symptomatic CAA) and WMH volume (in presymptomatic CAA) are the most promising markers to track short-term progression in future trials.
Conflict of interest statement
M.C. van der Plas and E.A. Koemans report no disclosures. M.R. Schipper reports independent support from the TRACK D-CAA consortium, consisting of Alnylam, Biogen, the Dutch CAA foundation, Vereniging HCHWA-D, and researchers from Leiden, Boston, and Perth. S. Voigt, I. Rasing, R.G.J. van der Zwet, K. Kaushik, R. van Dort, S. Schriemer, T.W. van Harten, E. van Zwet, and E.S. van Etten report no disclosures. M.J.P. van Osch reports support by a NWO-VICI grant (016.160.351) and a NWO-Human Measurement Models 2.0 grant (18969) as well as support from the Dutch Research Council (NWO), European Community, the Dutch Heart Foundation, and the Dutch Brain Foundation. G.M. Terwindt reports independent support from the Dutch Research Council (NWO), European Community, the Dutch Heart Foundation, the Dutch Brain Foundation, and the Dutch CAA foundation. M. van Walderveen reports no disclosures. M.J.H. Wermer reports independent support from the Dutch Research Council (NWO) ZonMw (VIDI grant 91717337), the Netherlands Heart Foundation (Dekker grant 2016T86), and the Dutch CAA foundation. Go to
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
