Identification of a molecular resistor that controls UCP1-independent Ca2+ cycling thermogenesis in adipose tissue
- PMID: 40199326
- PMCID: PMC12137002
- DOI: 10.1016/j.cmet.2025.03.009
Identification of a molecular resistor that controls UCP1-independent Ca2+ cycling thermogenesis in adipose tissue
Abstract
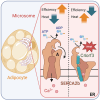
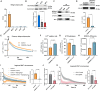
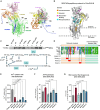
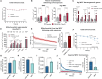
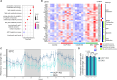
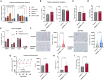
Adipose tissue thermogenesis contributes to energy balance via mitochondrial uncoupling protein 1 (UCP1) and UCP1-independent pathways. Among UCP1-independent thermogenic mechanisms, one involves Ca2+ cycling via SERCA2b in adipose tissue; however, the underlying molecular basis remains elusive. Here, we report that an endoplasmic reticulum (ER) membrane-anchored peptide, C4orf3 (also known as another regulin [ALN]), uncouples SERCA2b Ca2+ transport from its ATP hydrolysis, rendering the SERCA2b-C4orf3 complex exothermic. Loss of C4orf3/ALN improved the energetic efficiency of SERCA2b-dependent Ca2+ transport without affecting SERCA2 expression, thereby reducing adipose tissue thermogenesis and increasing the adiposity of mice. Notably, genetic depletion of C4orf3 resulted in compensatory activation of UCP1-dependent thermogenesis following cold challenge. We demonstrated that genetic loss of both C4orf3 and Ucp1 additively impaired cold tolerance in vivo. Together, this study identifies C4orf3 as the molecular resistor to SERCA2b-mediated Ca2+ import that plays a key role in UCP1-independent thermogenesis and energy balance.
Keywords: Ca(2+) cycling; UCP1-independent; energy balance; obesity; thermogenesis.
Crown Copyright © 2025. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Joule J.P. III. On the mechanical equivalent of heat. Philos. Trans. R. Soc. Lond. 1850;140:61–82. doi: 10.1098/rstl.1850.0004. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

