The evolution of AKAPs and emergence of PKA isotype selective anchoring determinants
- PMID: 40199400
- PMCID: PMC12083921
- DOI: 10.1016/j.jbc.2025.108480
The evolution of AKAPs and emergence of PKA isotype selective anchoring determinants
Abstract
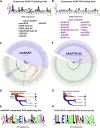
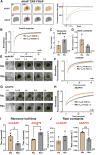
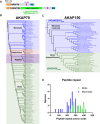
Cyclic AMP is a versatile signaling molecule utilized throughout the eukaryotic domain. A frequent use is to activate protein kinase A (PKA), a serine/threonine kinase that drives many physiological responses. Spatiotemporal organization of PKA occurs though association with A-kinase anchoring proteins (AKAPs). Sequence alignments and phylogenetic analyses trace the evolution of PKA regulatory (R) and catalytic (C) subunits and AKAPs from the emergence of metazoans. AKAPs that preferentially associate with the type I (RI) or type II (RII) regulatory subunits diverged at the advent of the vertebrate clade. Type I PKA anchoring proteins including smAKAP contain an FA motif at positions 1 and 2 of their amphipathic binding helices. Fluorescence recovery after photobleaching measurements indicate smAKAP preferentially associates with RI (T 1/2. 4.37 ± 1.2 s; n = 3) as compared to RII (T 1/2. 2.19 ± 0.5 s; n = 3). Parallel studies measured AKAP79 recovery half times of 8.74 ± 0.3 s (n = 3) for RI and 14.42 ± 2.1 s (n = 3) and for RII, respectively. Introduction of FA and AF motifs at either ends of the AKAP79 helix biases the full-length anchoring protein toward type I PKA signaling to reduce corticosterone release from adrenal cells by 61.5 ± 0.8% (n = 3). Conversely, substitution of the YA motif at the beginning of the smAKAP helix for a pair of leucine's abrogates RI anchoring. Thus, AKAPs have evolved from the base of the metazoan clade into specialized type I and type II PKA anchoring proteins.
Keywords: AKAP; amphipathic helix; evolution; protein kinase A; signal transduction.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interests The authors declare that they have no conflicts of interests with the contents of this article.
Figures





References
-
- Krebs E.G., Blumenthal D.K., Edelman A.M., Hales C.N. In: Mechanisms of Receptor Regulation. Crooke S.T., Poste G., editors. Plenum; New York: 1985. The functions of the cAMP-dependent protein kinase; pp. 324–367.
-
- Bos J.L. Epac: a new cAMP target and new avenues in cAMP research. Nat. Rev. Mol. Cell Biol. 2003;4:733–738. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

