Endothelial IGF- 1R deficiency disrupts microvascular homeostasis, impairing skeletal muscle perfusion and endurance: implications for age-related sarcopenia
- PMID: 40199795
- PMCID: PMC12181582
- DOI: 10.1007/s11357-025-01653-2
Endothelial IGF- 1R deficiency disrupts microvascular homeostasis, impairing skeletal muscle perfusion and endurance: implications for age-related sarcopenia
Abstract
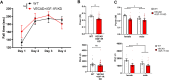
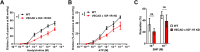
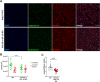
Aging is associated with a progressive decline in circulating insulin-like growth factor- 1 (IGF- 1) levels in humans, which has been implicated in the pathogenesis of sarcopenia. IGF- 1 is an anabolic hormone that plays a dual role in maintaining skeletal muscle health, acting both directly on muscle fibers to promote growth and indirectly by supporting the vascular network that sustains muscle perfusion. However, the microvascular consequences of IGF- 1 deficiency in aging muscle remain poorly understood. To elucidate how impaired IGF- 1 input affects skeletal muscle vasculature, we examined the effects of endothelial-specific IGF- 1 receptor (IGF- 1R) deficiency using a mouse model of endothelial IGF- 1R knockdown (VE-Cadherin-CreERT2/Igf1rf/f mice). These mice exhibited significantly reduced skeletal muscle endurance and attenuated hyperemic response to acetylcholine, an endothelium-dependent vasodilator. Additionally, they displayed microvascular rarefaction and impaired nitric oxide-dependent vasorelaxation, indicating a significant decline in microvascular health in skeletal muscle. These findings suggest that endothelial IGF- 1R signaling is critical for maintaining microvascular integrity, muscle perfusion, and function. Impaired IGF- 1 input to the microvascular endothelium may contribute to reduced muscle blood flow and exacerbate age-related sarcopenia. Enhancing vascular health by modulating IGF- 1 signaling could represent a potential therapeutic strategy to counteract age-related muscle decline.
Keywords: Aging; Claudication; Endothelial dysfunction; IGF- 1; IGF- 1R; Insulin-like growth factor- 1; Microvasculature; Sarcopenia; Skeletal muscle; Vascular function.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: N/A. Consent for publication: N/A. Competing interest: Dr. Adam Nyul-Toth, Dr. Roland Patai, Dr. Tamas Csipo, Dr. Anna Csiszar, Dr. Holly Van Remmen, Dr. Andriy Yabluchanskiy, and Dr. Stefano Tarantini serve as Associate Editors for GeroScience. Dr. Zoltan Ungvari serves as Editor-in-Chief for GeroScience and has personal relationships with individuals involved in the submission of this paper.
Figures





References
-
- Wiedmer P, Jung T, Castro JP, Pomatto LCD, Sun PY, Davies KJA, Grune T. Sarcopenia - molecular mechanisms and open questions. Ageing Res Rev. 2021;65: 101200. 10.1016/j.arr.2020.101200. - PubMed
-
- Sayer AA, Cooper R, Arai H, Cawthon PM, Ntsama Essomba MJ, Fielding RA, Grounds MD, Witham MD, Cruz-Jentoft AJ. Sarcopenia Nat Rev Dis Primers. 2024;10:68. 10.1038/s41572-024-00550-w. - PubMed
-
- Damluji AA, Alfaraidhy M, AlHajri N, Rohant NN, Kumar M, Al Malouf C, Bahrainy S, Ji Kwak M, Batchelor WB, Forman DE, Rich MW, Kirkpatrick J, Krishnaswami A, Alexander KP, Gerstenblith G, Cawthon P, deFilippi CR, Goyal P. Sarcopenia and cardiovascular diseases. Circulation. 2023;147:1534–53. 10.1161/CIRCULATIONAHA.123.064071. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

