Molecular subtyping of hypertensive disorders of pregnancy
- PMID: 40199872
- PMCID: PMC11978969
- DOI: 10.1038/s41467-025-58157-y
Molecular subtyping of hypertensive disorders of pregnancy
Abstract
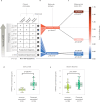
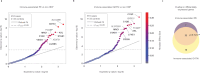
Hypertensive disorders of pregnancy (HDP), including preeclampsia, affect 1 in 6 pregnancies, are major contributors to maternal morbidity and mortality, yet lack precision medicine strategies. Analyzing transcriptomic data from a prospectively-collected diverse cohort (n = 9102), this study reveals distinct RNA subtypes in maternal blood, reclassifying clinical HDP phenotypes like early/late-onset preeclampsia. The placental gene PAPPA2 strongly predicts the most severe forms of preeclampsia in individuals without pre-existing high risk factors, months before symptoms, and its overexpression correlates with earlier delivery in a dose-dependent manner. Further, molecular subtypes characterized by immune genes are upregulated in less severe forms of HDP. These results reclassify HDP clinical phenotypes into two distinct molecular subtypes, placental-associated or immune-associated. Validation performance for placental-associated HDP yields an AUC of 0.88 in the advanced maternal age population without pre-existing high risk factors. Molecular subtypes create new opportunities to apply precision-based medicine in maternal health.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.A.E., E.P.S.G, N.D.-B., A.B.M., M. Reddy, A.K., J.L., I.A., K.M., H.C., F.S., R.N., T.F.M., S.R.Q., M.A.D., C.H., M.L., E.N., M.J. and M. Rasmussen have an equity interest in Mirvie. Mirvie Inc. provided funding for the study. The following patents and patent applications cover various aspects described in the manuscript, including detecting presence or elevated risk of pregnancy complications such as preeclampsia in a pregnant subject based on differential expression of RNA markers: US Patent No 11,208,693 (Mirvie Inc, M.J., E.N., M.Rasmussen, F.S., M.Reddy), US Application No. 18/167,322 (Mirvie Inc, M.J., E.N., M.Rasmussen, F.S., M.Reddy, E.P.S.G, A.K., R.N., M.L.), and International Application No PCT/US2024/027,444 (Mirvie Inc, M.J., E.N., M.Rasmussen, F.S., M.Reddy, E.P.S.G., A.K., R.N., M.L., N.D.-B.). The remaining authors declare no competing interests.
Figures


References
-
- Motomura, K., Morita, H., Naruse, K., Saito, H. & Matsumoto, K. Implication of viruses in the etiology of preeclampsia. Am. J. Reprod. Immunol.91, e13844 (2024). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

