An Oryza-specific histone H4 variant predisposes H4 lysine 5 acetylation to modulate salt stress responses
- PMID: 40200022
- PMCID: PMC7617672
- DOI: 10.1038/s41477-025-01974-2
An Oryza-specific histone H4 variant predisposes H4 lysine 5 acetylation to modulate salt stress responses
Abstract
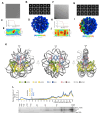
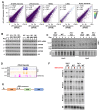
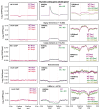
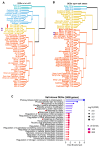
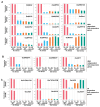
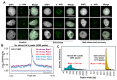
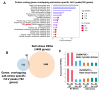
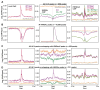
Paralogous variants of canonical histones guide accessibility to DNA and function as additional layers of genome regulation. Across eukaryotes, the mechanism of action and functional significance of several variants of core histones are well known except those of histone H4. Here we show that a variant of H4 (H4.V) expressing tissue-specifically among Oryza members mediated specific epigenetic changes contributing to salt tolerance. H4.V was incorporated into specific heterochromatic sites, where it blocked the deposition of active histone marks. Stress-dependent redistribution of H4.V enabled the incorporation of acetylated H4 lysine 5 (H4K5ac) in the gene bodies. The misexpression of H4.V led to defects in reproductive development and in mounting salt stress responses. H4.V formed homotypic nucleosomes and mediated these alterations by conferring distinct molecular properties to the nucleosomes, as seen with cryo electron microscopy structures and biochemical assays. These results reveal not only an H4 variant among plants but also a chromatin regulation that might have contributed to the adaptation of semi-aquatic Oryza members.
© 2025. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures









References
-
- Kornberg RD. Chromatin structure: a repeating unit of histones and DNA. Science. 1974;184:868–871. - PubMed
-
- Hocher A, Laursen SP, Radford P, Tyson J, Lambert C, Stevens KM, Picardeau M, Sockett RE, Luger K, Warnecke T. Histone-organized chromatin in bacteria. bioRxiv. 2023 doi: 10.1101/2023.01.26.525422. - DOI
-
- Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997;389:251–260. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
- 202811/WT_/Wellcome Trust/United Kingdom
- BT/IN/Swiss/47/JGK/2018-19/Department of Biotechnology, Ministry of Science and Technology (DBT)
- CRG/2023/003849/DST | Science and Engineering Research Board (SERB)
- RTI 4006 (1303/3/2019/R&D-II/DAE/4749 dated 16.7.2020)/Department of Atomic Energy, Government of India (DAE)
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources

