The effective-compound compatibility of JHF inhibits fibroblast activation in pulmonary fibrosis by enhancing PINK1/PARK2-mediated mitophagy
- PMID: 40200051
- PMCID: PMC11978889
- DOI: 10.1038/s41598-025-95175-8
The effective-compound compatibility of JHF inhibits fibroblast activation in pulmonary fibrosis by enhancing PINK1/PARK2-mediated mitophagy
Abstract
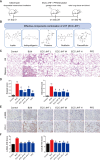
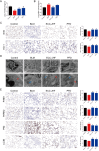
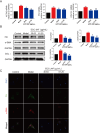
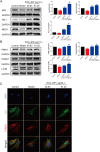
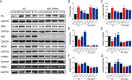
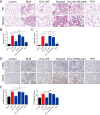
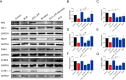
This work aimed to elucidate the anti-PF mechanism of ECC-JHF.The effects of ECC-JHF on lung fibrosis and fibroblast activation were investigated by establishing a BLM-induced PF rat model and a transforming growth factor-beta (TGF-β)-induced fibroblast activation model. Furthermore, the effects of ECC-JHF on Nrf2 signaling and mitophagy were explored both in vivo and in vitro. In the PF model rats, ECC-JHF mitigated pathological damage, reduced collagen deposition, decreased levels of malondialdehyde (MDA) and P62, and increased levels of total superoxide dismutase (T-SOD) as well as the expression of Nrf2, HO-1, PINK1, PARK2, and LC3B in lung tissues. These results suggest that the anti-PF mechanism of ECC-JHF may be associated with the inhibition of oxidative stress and the enhancement of mitophagy. The medium dose of ECC-JHF and pirfenidone were similar in improving pulmonary fibrosis in rats. In the TGF-β-induced lung fibroblast activation, ECC-JHF inhibited fibroblast activation by downregulating the levels of fibronectin, alpha-smooth muscle actin (α-SMA), and collagen I. Additionally, ECC-JHF upregulated the level of Nrf2 and its target proteins, including HO-1 and NQO1, as well as mitophagy-related proteins PINK1, PARK2, and LC3B. This led to an increase in the co-localization of TOM20 and LC3, thereby enhancing mitochondrial autophagy. The application of Nrf2 siRNA and Nrf2 inhibitors significantly diminished the effects of ECC-JHF on Nrf2 signaling, PINK1/PARK2-mediated mitophagy, and fibroblast activation. ECC-JHF exerts a protective effect against PF by suppressing fibroblast activation through the upregulation of Nrf2 and PINK1/PARK2-mediated mitophagy, it provides a new target and strategy for the treatment of pulmonary fibrosis.
Keywords: Effective-compound combination; Fibroblasts activation; Mitophagy; Nrf2; Pulmonary fibrosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: The Sprague-Dawley rats (license number: SCXK (Beijing) 2016-0006) were supplied by Beijing Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). All experimental procedures received approval from the First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, China (YFYDW2017013). Consent for publication: In this study, all participants provided written informed consent for their data to be used in publications.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

