A tardive dyskinesia drug target VMAT-2 participates in neuronal process elongation
- PMID: 40200061
- PMCID: PMC11978964
- DOI: 10.1038/s41598-025-97308-5
A tardive dyskinesia drug target VMAT-2 participates in neuronal process elongation
Abstract
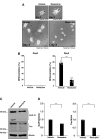
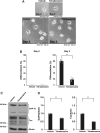
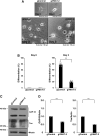
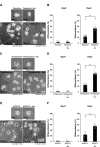
Tardive dyskinesia involves involuntary movements of body parts and is often observed in individuals taking antipsychotics for extended periods. Initial treatment strategies include reducing medication dosage, switching medications, or using drugs to suppress symptoms. One of the therapeutic targets for tardive dyskinesia is vesicular monoamine transporter-2 (VMAT-2, also known as solute carrier family 18 member A2 [SLC18A2]), which functions as an energy-dependent transporter of monoamines. The therapeutic drugs are used during adulthood, when neurons are maturing. For the first time, we report that treatment with a chemical VMAT-2 inhibitor reduces neuronal process elongation, a phenomenon commonly observed during development. Treatment with the inhibitors reserpine or tetrabenazine decreased process elongation in primary cortical neurons, and similar results were obtained in N1E-115 neuronal model cells undergoing process elongation. Knockdown of VMAT-2 using clustered regularly interspaced short palindromic repeat (CRISPR)/Cas13-fitted guide RNA also reduced process elongation. However, treatment with reserpine or tetrabenazine did not affect the morphology of mature processes. Notably, treatment with hesperetin, a citrus flavonoid with neuroprotective effects, was able to restore the reduced process elongation induced by these inhibitors or VMAT-2 knockdown. The underlying molecular mechanism appeared to involve neuronal differentiation-related Akt kinase signaling. These results suggest that VMAT-2, as a drug target for tardive dyskinesia, plays a key role in process elongation and that some inhibitory effects of VMAT-2-targeted drugs on its elongation may be mitigated by co-administering a neuroprotective molecule.
Keywords: CRISPR-Cas13; Elongation; Hesperetin; Reserpine; Tardive dyskinesia; Tetrabenazine; VMAT-2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Deuterium Tetrabenazine for Tardive Dyskinesia.Clin Schizophr Relat Psychoses. 2018 Jan;11(4):214-220. doi: 10.3371/CSRP.CUPR.010318. Clin Schizophr Relat Psychoses. 2018. PMID: 29341821 Review.
-
Mechanism of action of vesicular monoamine transporter 2 (VMAT2) inhibitors in tardive dyskinesia: reducing dopamine leads to less "go" and more "stop" from the motor striatum for robust therapeutic effects.CNS Spectr. 2018 Feb;23(1):1-6. doi: 10.1017/S1092852917000621. Epub 2017 Dec 18. CNS Spectr. 2018. PMID: 29249207 Review.
-
Valbenazine for the treatment of tardive dyskinesia.Drugs Today (Barc). 2016 Dec;52(12):665-672. doi: 10.1358/dot.2016.52.12.2570977. Drugs Today (Barc). 2016. PMID: 28276538 Review.
-
VMAT2 Inhibitors for Tardive Dyskinesia-Practice Implications.J Pharm Pract. 2019 Aug;32(4):450-457. doi: 10.1177/0897190018756512. Epub 2018 Feb 18. J Pharm Pract. 2019. PMID: 29455579 Review.
-
Deutetrabenazine in the treatment of tardive dyskinesia.Neurodegener Dis Manag. 2019 Apr;9(2):59-71. doi: 10.2217/nmt-2018-0042. Epub 2019 Jan 31. Neurodegener Dis Manag. 2019. PMID: 30702019
References
-
- Bashir, H. H. & Jankovic, J. Treatment of tardive dyskinesia. Neurol. Clin.38, 379–396 (2020). - PubMed
-
- Ismail, O., Albdour, K., Jaber, Y., Jaber, K. & Alsaras, A. Efficacy and safety of different Pharmacological interventions in the treatment of tardive dyskinesia: A systematic review and network meta-analysis. Eur. J. Clin. Pharmacol.80, 1471–1482 (2024). - PubMed
-
- Correll, C. U. & Carbon, M. A new class of VMAT-2 inhibitors for tardive dyskinesia. Lancet Psychiatry4, 574–575 (2017). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

