PCYT2 mediates ovarian epithelial cancer metastasis by regulating cell membrane fluidity through the AMPK/FOXO1 signalling pathway
- PMID: 40200068
- PMCID: PMC11978741
- DOI: 10.1038/s41598-025-96405-9
PCYT2 mediates ovarian epithelial cancer metastasis by regulating cell membrane fluidity through the AMPK/FOXO1 signalling pathway
Abstract
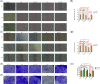
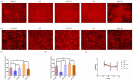
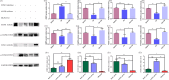
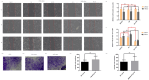
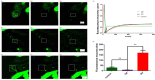
This study investigates the role of phosphatidylethanolamine cytidylyltransferase 2 (PCYT2) in ovarian epithelial cancer, specifically examining its effects on cell migration and membrane fluidity. To achieve this, we will examine how the AMPK and FOXO1 pathways regulate these processes. Our analysis revealed a significant upregulation of PCYT2 expression in metastatic ovarian cancer tissues compared to primary cancer sites, which correlates with altered membrane fluidity. Our data indicate that PCYT2 is essential for modulating the invasive characteristics of ovarian cancer cells. It does this by regulating the expression levels of AMPK and FOXO1, suggesting its role as an upstream regulator in this signaling pathway. Experiments that either inhibit or enhance PCYT2 activity suggest that it may influence cancer cell infiltration by changing membrane fluidity. These findings provide valuable insights into the molecular mechanisms of ovarian cancer metastasis and highlight PCYT2 as a promising therapeutic target. Future research should validate these findings in larger cohort studies, and also explore the therapeutic potential of targeting PCYT2 in ovarian cancer treatment. In conclusion, although there have been substantial advancements in ovarian cancer therapies, the intricate nature of its metastatic behavior remains a major challenge. Our research clearly demonstrates the critical role of PCYT2, urging the scientific community to deepen their understanding of its involvement in cancer progression and to develop innovative treatment strategies.
Keywords: AMPK/FOXO1 signalling pathway; Cell membrane fluidity; Metastasis.; Ovarian epithelial cancer; PCYT2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Informed consent statement: Written informed consent, covering the use of clinical data and tumour tissue for research purposes, was obtained from all participating patients. Institutional review board statement: The study protocol received approval from the Institutional Review Board (IRB) of Qingpu Branch of Zhongshan Hospital (IRB No. 2022-39) and was conducted in accordance with the ethical principles outlined in the 1975 Declaration of Helsinki (6th revision, 2008). Competing interests: The authors declare no competing interests.
Figures


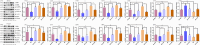
Similar articles
-
Prognostic implications of forkhead box protein O1 (FOXO1) and paired box 3 (PAX3) in epithelial ovarian cancer.BMC Cancer. 2019 Dec 10;19(1):1202. doi: 10.1186/s12885-019-6406-6. BMC Cancer. 2019. PMID: 31823759 Free PMC article.
-
MiR-27a promotes EMT in ovarian cancer through active Wnt/𝜷-catenin signalling by targeting FOXO1.Cancer Biomark. 2019;24(1):31-42. doi: 10.3233/CBM-181229. Cancer Biomark. 2019. PMID: 30614794
-
Cysteine-Rich Intestinal Protein 1 Served as an Epithelial Ovarian Cancer Marker via Promoting Wnt/β-Catenin-Mediated EMT and Tumour Metastasis.Dis Markers. 2021 Aug 6;2021:3566749. doi: 10.1155/2021/3566749. eCollection 2021. Dis Markers. 2021. PMID: 34413913 Free PMC article.
-
Propofol inhibits proliferation and cisplatin resistance in ovarian cancer cells through regulating the microRNA‑374a/forkhead box O1 signaling axis.Mol Med Rep. 2020 Mar;21(3):1471-1480. doi: 10.3892/mmr.2020.10943. Epub 2020 Jan 16. Mol Med Rep. 2020. PMID: 32016462 Free PMC article.
-
The Role of microRNAs in Epithelial Ovarian Cancer Metastasis.Int J Mol Sci. 2020 Sep 25;21(19):7093. doi: 10.3390/ijms21197093. Int J Mol Sci. 2020. PMID: 32993038 Free PMC article. Review.
References
-
- McGowan, L. Pathology of the ovary. Curr. Opin. Obstet. Gynecol.3(4), 580–586 (1991). - PubMed
-
- Li, X. Q. et al. Angiotensin-converting enzyme-dependent intrarenal angiotensin II contributes to CTP: phosphoethanolamine cytidylyltransferase downregulation, mitochondrial membranous disruption, and reactive oxygen species overgeneration in diabetic tubulopathy. Antioxid. Redox Signal. (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

