Genetic and structural insights into the functional importance of the conserved gly-met-rich C-terminal tails in bacterial chaperonins
- PMID: 40200084
- PMCID: PMC11978752
- DOI: 10.1038/s42003-025-07927-x
Genetic and structural insights into the functional importance of the conserved gly-met-rich C-terminal tails in bacterial chaperonins
Abstract
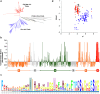
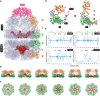
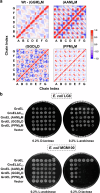
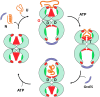
E. coli chaperonin GroEL forms nano-cages for protein folding. Although the chaperonin-mediated protein folding mechanism is well understood, the role of the conserved glycine and methionine-rich carboxy-terminal residues remains unclear. Bacteria with multiple chaperonins always retain at least one paralogue having the gly-met-rich C-terminus, indicating an essential conserved function. Here, we observed a stronger selection pressure on the paralogues with gly-met-rich C-termini, consistent with their ancestral functional importance. E. coli GroEL variants having mutations in their C-termini failed to functionally replace GroEL, suggesting the functional significance of the gly-met-rich C-termini. Further, our structural modelling and normal mode analysis showed that the C-terminal region shuttles between two cavity-specific conformations that correlate with the client-protein-binding apical domains, supporting C-termini's role in client protein encapsulation. Therefore, employing phylogenetic, genetic, and structural tools, we demonstrate that the gly-met-rich C-termini are functionally significant in chaperonin-mediated protein folding function. Owing to the pathogenic roles of the chaperonins having non-canonical C-termini, future investigations on the client protein selectivity will enable understanding the disease-specific client protein folding pathways and treatment options.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
The C-terminal tails of the bacterial chaperonin GroEL stimulate protein folding by directly altering the conformation of a substrate protein.J Biol Chem. 2014 Aug 15;289(33):23219-23232. doi: 10.1074/jbc.M114.577205. Epub 2014 Jun 25. J Biol Chem. 2014. PMID: 24970895 Free PMC article.
-
Structural features of the GroEL-GroES nano-cage required for rapid folding of encapsulated protein.Cell. 2006 Jun 2;125(5):903-14. doi: 10.1016/j.cell.2006.04.027. Cell. 2006. PMID: 16751100
-
Functional Differences between E. coli and ESKAPE Pathogen GroES/GroEL.mBio. 2021 Jan 12;12(1):e02167-20. doi: 10.1128/mBio.02167-20. mBio. 2021. PMID: 33436430 Free PMC article.
-
Reaction Cycle of Chaperonin GroEL via Symmetric "Football" Intermediate.J Mol Biol. 2015 Sep 11;427(18):2912-8. doi: 10.1016/j.jmb.2015.04.007. Epub 2015 Apr 18. J Mol Biol. 2015. PMID: 25900372 Review.
-
Molecular chaperone GroEL/ES: unfolding and refolding processes.Biochemistry (Mosc). 2013 Dec;78(13):1405-14. doi: 10.1134/S0006297913130038. Biochemistry (Mosc). 2013. PMID: 24490731 Review.
References
-
- Horwich, A. L. & Fenton, W. A. Chaperonin-assisted protein folding: a chronologue. Q Rev. Biophys.53, e4 (2020). - PubMed
-
- Lund, P. A. Multiple chaperonins in bacteria- why so many? FEMS Microbiol Rev.33, 785–800 (2009). - PubMed
-
- Hayer-Hartl, M., Bracher, A. & Hartl, F. U. The GroEL-GroES Chaperonin machine: a nano-cage for protein folding. Trends Biochem Sci.41, 62–76 (2016). - PubMed
-
- Braig, K. et al. The crystal structure of the bacterial chaperonin GroEL at 2.8 A. Nature371, 578–586 (1994). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

