Clinical study of 37 cases of orbital melanoma
- PMID: 40200217
- PMCID: PMC11978098
- DOI: 10.1186/s12886-025-03897-0
Clinical study of 37 cases of orbital melanoma
Abstract
Background: To analyze the clinical features and prognosis of orbital melanoma.
Design: Retrospective observational case series.
Methods: A retrospective analysis of the electronic medical records, histopathology, imaging examinations, and follow-up information of 37 patients with orbital melanoma.
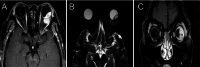
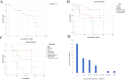
Results: The most common primary site was the conjunctiva, with half of the patients presenting with orbital involvement at the initial visit. The most common symptoms were globe displacement due to intraorbital mass, causing swelling of the eyelids and blurred vision. All patients underwent mass excision surgery. Rates of intraorbital soft tissue infiltration, nerve invasion, and bone destruction were 48.6%, 18.9%, and 13.5%, respectively. Five patients (13.5%) have distant metastases to the liver, bones, lungs, skin, and parotid lymph nodes. The Median Survival Time of the 13 deceased patients was 132 ± 24.88 months, with a 5-year survival rate of 78.4%±7.3%. The presence of nerve invasion showed a significant correlation with prognosis (P = 0.047 < 0.05), while age, gender, eye involvement, bone destruction, and intraorbital soft tissue infiltration showed no significant correlation with prognosis (P > 0.05). The expression of Ki-67 was negatively correlated with patient survival time and rate, where higher Ki-67 expression was associated with shorter survival time (r2=-0.267, r2=-0.067).
Conclusions: Treatment strategies for orbital melanoma should consider the tumor's invasive characteristics and Ki-67 expression levels to optimize treatment outcomes and improve patient survival rates. Furthermore, due to the significant impact of nerve involvement on prognosis, it is recommended that clinical focus on this factor be enhanced.
Keywords: Clinical presentation; Melanoma; Orbit; Prognosis; Treatment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The name of the ethics committee: Ethics Committee of Beijing Tongren Hospital, Capital Medical University Ethics No. TREC2023-KY061. Informed consent: has been obtained from all participants in the study. Consent for publication: Not Applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
[Surgical management of orbital malignant melanoma: our experience and a report of three cases].Klin Monbl Augenheilkd. 2007 Oct;224(10):794-8. doi: 10.1055/s-2007-963517. Klin Monbl Augenheilkd. 2007. PMID: 17952825 German.
-
Secondary orbital melanomas: analysis of 15 cases.J Craniomaxillofac Surg. 2000 Jun;28(3):148-52. doi: 10.1054/jcms.2000.0132. J Craniomaxillofac Surg. 2000. PMID: 10964550
-
Primary and secondary orbital melanomas: a clinical and prognostic study.Ophthalmic Plast Reconstr Surg. 1995 Sep;11(3):169-81. doi: 10.1097/00002341-199509000-00003. Ophthalmic Plast Reconstr Surg. 1995. PMID: 8541258
-
Primary orbital melanoma: A report of a case and comprehensive review of the literature.Orbit. 2021 Dec;40(6):461-469. doi: 10.1080/01676830.2020.1818265. Epub 2020 Sep 9. Orbit. 2021. PMID: 32900269 Free PMC article. Review.
-
Primary orbital melanoma: a case series and literature review.Orbit. 2018 Oct;37(5):352-357. doi: 10.1080/01676830.2017.1423354. Epub 2018 Feb 1. Orbit. 2018. PMID: 29388848 Review.
References
-
- Bonavolontà G, Strianese D, Grassi P, et al. An analysis of 2,480 space-occupying lesions of the orbit from 1976 to 2011. Ophthalmic Plast Reconstr Surg. 2013;29(2):79–86. - PubMed
-
- Delaney YM, Hague S, McDonald B. Aggressive primary orbital melanoma in a young white man with no predisposing ocular features. Arch Ophthalmol. 2004;122(1):118–21. - PubMed
-
- Esmaeli B, Sagiv O. Targeted Biological drugs and Immune check point inhibitors for locally advanced or metastatic cancers of the Conjunctiva, Eyelid, and Orbit. Int Ophthalmol Clin. 2019;59(2):13–26. - PubMed
Publication types
MeSH terms
Grants and funding
- No. DFL20190201/Natural Science Foundation of Beijing Municipality
- No. DFL20190201/Natural Science Foundation of Beijing Municipality
- No. DFL20190201/Natural Science Foundation of Beijing Municipality
- No. DFL20190201/Natural Science Foundation of Beijing Municipality
- 20220484218/Beijing Nova Program
- 20220484218/Beijing Nova Program
- 20220484218/Beijing Nova Program
- 20220484218/Beijing Nova Program
- No7222025/Beijing Municipal Administration of Hospitals' Ascent Plan
- No7222025/Beijing Municipal Administration of Hospitals' Ascent Plan
- No7222025/Beijing Municipal Administration of Hospitals' Ascent Plan
- No7222025/Beijing Municipal Administration of Hospitals' Ascent Plan
- ZR202306130003/Department of Science and Technology of Shandong Province
- ZR202306130003/Department of Science and Technology of Shandong Province
LinkOut - more resources
Full Text Sources
Medical

