TRIM22 governs tumorigenesis and protects against endometrial cancer-associated cachexia by inhibiting inflammatory response and adipose thermogenic activity
- PMID: 40200303
- PMCID: PMC11980105
- DOI: 10.1186/s40170-025-00386-2
TRIM22 governs tumorigenesis and protects against endometrial cancer-associated cachexia by inhibiting inflammatory response and adipose thermogenic activity
Abstract
Background: Endometrial cancer (EC) is one of the most common cancers in women, with a short overall survival and poor prognosis. Besides the biologically aggressive EC properties, Cancer-associated cachexia is the main factor. However, the detailed mechanism underlying EC-related cachexia and its harmful effects on EC progression and patient prognosis remains unclear.
Methods: For clinical specimen and the vitro experiment, we detected TRIM22 expression level, EC patients' survival time, EC cell functional change, and adipose thermogenic changes to identify the function of TRIM22 in EC progression, EC-associated cachexia, and their molecular mechanisms. Then, for the vivo experiment, we exploited the xenografts in mice to identify the function of TRIM22 again, and to screen the drug therapeutic schedule.
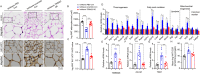
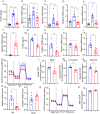
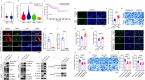
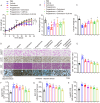
Results: Herein, we demonstrated that TRIM22 inhibited EC cell growth, invasion, and migration. Interleukin (IL)-6 mediated brown adipose tissue activation and white adipose tissue browning which induced EC-related cachexia. TRIM22 suppressed the EC cells' secretion of IL-6, and IL-6 mediated EC-related cachexia. Mechanistically, TRIM22 inhibited EC progression by suppressing the nucleotide-binding oligomerization domain 2(NOD2)/nuclear factor-kappaB (NF-κB) signaling pathway, with the purpose of impeding the production of IL-6. Moreover, we revealed that TRIM22 inhibited EC-associated cachexia by suppressing the IL-6/IL-6 receptor (IL-6R) signaling pathway. Therapeutically, we demonstrated that combination treatment with a TRIM22 inducer (progesterone) and a thermogenic inhibitor (IL-6R antibody) synergistically augmented the antitumor efficacy of carbotaxol (carboplatin and paclitaxel), in vivo.
Conclusion: Our data reveals that TRIM22-EC-IL-6-cachexia cross-communication has important clinical relevance and that the use of combined therapy holds great promise for enhancing the efficacy of anti-ECs. (Fig. graphical abstract).
Keywords: Antitumor therapy; Cancer-associated cachexia; Endometrial cancer; Tripartite motif-containing 22(TRIM22).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: This study was approved by the research ethics committee of Fujian Medical University Union Hospital (Fuzhou, China), and carried out under the World Medical Association Declaration of Helsinki. Consent for publication: The authors confirm that they have obtained written consent from each patient to publish the manuscript. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
TRIM22 inhibits endometrial cancer progression through the NOD2/NF‑κB signaling pathway and confers a favorable prognosis.Int J Oncol. 2020 May;56(5):1225-1239. doi: 10.3892/ijo.2020.5004. Epub 2020 Mar 4. Int J Oncol. 2020. PMID: 32319602 Free PMC article.
-
Knockdown of TRIM22 Relieves Oxygen-Glucose Deprivation/Reoxygenation-Induced Apoptosis and Inflammation Through Inhibition of NF-κB/NLRP3 Axis.Cell Mol Neurobiol. 2021 Mar;41(2):341-351. doi: 10.1007/s10571-020-00855-w. Epub 2020 Apr 25. Cell Mol Neurobiol. 2021. PMID: 32335773 Free PMC article.
-
TRIM22 activates NF-κB signaling in glioblastoma by accelerating the degradation of IκBα.Cell Death Differ. 2021 Jan;28(1):367-381. doi: 10.1038/s41418-020-00606-w. Epub 2020 Aug 19. Cell Death Differ. 2021. PMID: 32814880 Free PMC article.
-
Role of interleukin-6 in cachexia: therapeutic implications.Curr Opin Support Palliat Care. 2014 Dec;8(4):321-7. doi: 10.1097/SPC.0000000000000091. Curr Opin Support Palliat Care. 2014. PMID: 25319274 Free PMC article. Review.
-
Lipid metabolism in cancer cachexia.Ann Palliat Med. 2019 Jan;8(1):13-23. doi: 10.21037/apm.2018.10.01. Epub 2018 Oct 30. Ann Palliat Med. 2019. PMID: 30525767 Review.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Global Cancer. Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. 10.3322/caac.21660. - PubMed
-
- Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet. 2022;399(10333):1412–28. 10.1016/S0140-6736(22)00323-3. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

