Formation of the toxic furan metabolite 2-butene-1,4-dial through hemin-induced degradation of 2,4-alkadienals in fried foods
- PMID: 40200382
- PMCID: PMC11978195
- DOI: 10.1186/s41021-025-00330-2
Formation of the toxic furan metabolite 2-butene-1,4-dial through hemin-induced degradation of 2,4-alkadienals in fried foods
Abstract
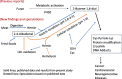
Background: The mechanism of protein modification by 2,4-alkadienals (ADE), lipid peroxidation products prevalent in fried foods, was investigated through model reactions.
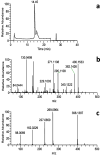
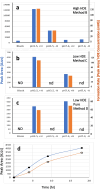
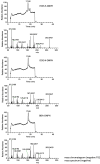
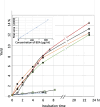
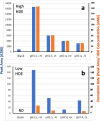
Results: A mixture of 2,4-heptadienal (HDE) and hemin was initially incubated at pH 3.0-7.4, followed by treatment with acetyl-cysteine (AcCys) and acetyl-lysine (AcLys) at pH 7.4. Analysis via HPLC revealed a product with a characteristic UV spectrum as the primary peak. This product was identified as an AcCys-pyrrole-AcLys (CPL) crosslink derived from AcCys, 2-butene-1,4-dial (BDA), and AcLys. Increasing the HDE concentration in the initial reaction led to maximum CPL formation at pH 3.5 in the presence of hemin. Lowering the HDE concentration with a higher Cys/HDE ratio resulted in CPL formation, which was observed at pH 7.4 and 3.5 in the presence of hemin. Upon incubation of ADE and hemin at pH 3.0-3.5, BDA was directly identified as 2,4-dinitrophenylhydrazone. BDA was also detected in the 2,4-decadienal reaction mixture. Additionally, a notable propensity for high BDA-dC adduct formation with hemin under acidic conditions was observed, consistent with the results of CPL assay and BDA-2,4-dinitrophenylhydrazone analysis.
Conclusions: 1) BDA is efficiently generated from ADE in the presence of hemin under gastric conditions, and 2) BDA-derived CPL can also form under physiological conditions (pH 7.4) through the interaction of ADE, hemin, Cys, and Lys. BDA is recognized as the primary reactive metabolite of the suspected carcinogen furan (IARC, 2B). Given that human intake of ADE exceeds that of furan and acrylamide (IARC 2A) by several orders of magnitude, and the estimated hemin concentration in the stomach post-meal is comparable to the present study, a substantial amount of BDA may form in the stomach following consumption of fried foods and meat. The risk assessment of ADE warrants a thorough re-evaluation, based on the toxicity mechanism of BDA.
Keywords: 2,4-decadienal; 2,4-heptadienal; 2-butene-1,4-dial; Cysteine-pyrrole-lysine; Fried foods; Furan; Protein crosslink.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Characterization of amino acid and glutathione adducts of cis-2-butene-1,4-dial, a reactive metabolite of furan.Chem Res Toxicol. 1997 Aug;10(8):866-74. doi: 10.1021/tx9700174. Chem Res Toxicol. 1997. PMID: 9282835
-
New insight into the molecular mechanism of protein cross-linking induced by cis-2-butene-1,4-dial, the metabolite of furan: Formation of 2-substituted pyrrole cross-links involving the cysteine and lysine residues.Bioorg Chem. 2022 Aug;125:105852. doi: 10.1016/j.bioorg.2022.105852. Epub 2022 May 5. Bioorg Chem. 2022. PMID: 35551004
-
Degraded protein adducts of cis-2-butene-1,4-dial are urinary and hepatocyte metabolites of furan.Chem Res Toxicol. 2009 Jun;22(6):997-1007. doi: 10.1021/tx800377v. Chem Res Toxicol. 2009. PMID: 19441776 Free PMC article.
-
A comprehensive review of furan in foods: From dietary exposures and in vivo metabolism to mitigation measures.Compr Rev Food Sci Food Saf. 2023 Mar;22(2):809-841. doi: 10.1111/1541-4337.13092. Epub 2022 Dec 21. Compr Rev Food Sci Food Saf. 2023. PMID: 36541202 Review.
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
References
-
- Dangal A, Tahergorabi R, Acharya DR, Timsina P, Rai K, Dahal S, Giuffrè AM. Review on deep-fat fried foods: Physical and chemical attributes, and consequences of high consumption. Eur Food Res Technol. 2024;250(6):1537–50.
-
- Grinshtein N, Bamm VV, Tsemakhovich VA, Shaklai N. Mechanism of low-density lipoprotein oxidation by hemoglobin-derived iron. Biochemistry. 2003;42(23):6977–85. - PubMed
LinkOut - more resources
Full Text Sources

