The Fourth International Consensus Guidelines on the Management of Cytomegalovirus in Solid Organ Transplantation
- PMID: 40200403
- PMCID: PMC12180710
- DOI: 10.1097/TP.0000000000005374
The Fourth International Consensus Guidelines on the Management of Cytomegalovirus in Solid Organ Transplantation
Figures



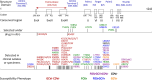
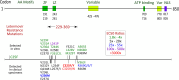
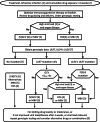
References
-
- Kotton CN, Kumar D, Caliendo AM, et al. ; Transplantation Society International CMV Consensus Group. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795. - PubMed
-
- Kotton CN, Kumar D, Caliendo AM, et al. ; Transplantation Society International CMV Consensus Group. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360. - PubMed
-
- Kotton CN, Kumar D, Caliendo AM, et al. ; The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102:900–931. - PubMed
LinkOut - more resources
Full Text Sources

