Single-Dose, Intravenous, and Oral Pharmacokinetics of Isavuconazole in Dogs
- PMID: 40200720
- PMCID: PMC12257257
- DOI: 10.1111/jvp.13510
Single-Dose, Intravenous, and Oral Pharmacokinetics of Isavuconazole in Dogs
Abstract
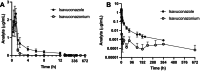
Isavuconazole, a triazole antifungal used in humans for invasive fungal infections, may be effective for treating canine fungal infections, although data on its use in dogs is limited. This study aimed to determine the pharmacokinetics and safety of a single dose of isavuconazole in dogs, administered both intravenously and orally. Six healthy dogs received 186 mg isavuconazonium sulfate in a crossover design, with blood samples collected over 28 days and an 8-week washout period. Plasma isavuconazole and isavuconazonium concentrations were measured by liquid chromatography/mass spectrometry, and pharmacokinetic parameters were determined by non-compartmental analysis. Isavuconazole was well tolerated, with key findings including intravenous clearance at 350 ± 112 mL/kg/h, volume of distribution at steady state at 9.8 ± 4.5 L/kg, and a terminal half-life of 90 ± 44 h. For oral administration, the maximum concentration was 0.60 ± 0.27 μg/mL, time to maximum concentration was 6.73 ± 2.45 h, terminal half-life was 125 ± 80 h, and the area under the curve was 7.44 ± 2.39 μg h/mL. Oral bioavailability was 81.4% ± 12.8%. These results suggest isavuconazole has a long half-life in dogs and is well absorbed orally when administered in the fasted state. Further studies are warranted to establish a therapeutic regimen in dogs.
Keywords: antifungal; aspergillosis; dog; isavuconazole; pharmacokinetics; triazole.
© 2025 The Author(s). Journal of Veterinary Pharmacology and Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
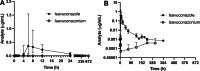
References
-
- Boothe, D. M. 2011. Small Animal Clinical Pharmacology and Therapeutics. 2nd ed. Elsevier/Saunders.
-
- Cresemba . 2022. Cresemba (Isavuconazonium Sulfate) [Package Insert]. Astrellas Pharma US, Inc.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

