Co-expression of HSV-1 ICP34.5 enhances the expression of gene delivered by self-amplifying RNA and mitigates its immunogenicity
- PMID: 40200732
- PMCID: PMC12226409
- DOI: 10.1002/2211-5463.70036
Co-expression of HSV-1 ICP34.5 enhances the expression of gene delivered by self-amplifying RNA and mitigates its immunogenicity
Abstract
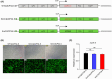
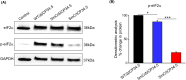
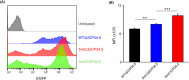
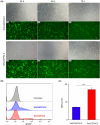
Self-amplifying RNA (saRNA) vectors have garnered significant attention for their potential in transient recombinant protein expression and vaccination strategies. These vectors are notable for their safety and the ability to produce high levels of protein from minimal input templates, offering a promising avenue for gene therapy applications. Despite their advantages, saRNA vectors face a critical challenge in their propensity to trigger a robust innate immune response. The presence of double-stranded RNA intermediates during saRNA replication activates pattern recognition receptors (PRRs), leading to the activation of protein kinase R (PKR) and interferon (IFN) signaling, which can result in a general translational shutdown within the host cell. To mitigate the stimulatory effects on PRRs and enhance the translation efficiency of saRNA, this study employs the saRNA-encoding HSV-1 neurovirulence protein ICP34.5, which is known for its ability to counteract the effects of PKR activation, potentially improving the translation efficiency of saRNA. It was shown that saRNA-encoding ICP34.5 clearly mediated the eukaryotic initiation factor 2 alpha subunit (eIF2α) dephosphorylation and significant suppression of innate immune responses in vitro, leading to enhanced expression of saRNA-encoded genes. The application of ICP34.5 incorporating saRNA vectors offers a more efficient and cost-effective solution for the production of proteins and the development of vaccines. This strategy could revolutionize the fields where saRNA utilization is envisioned, particularly in neurotropic disease applications where HSV-1 proteins may offer additional benefits.
Keywords: ICP34.5; gene expression; innate immune response; saRNA; vaccines.
© 2025 The Author(s). FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
IFN alpha inducible protein 27 (IFI27) acts as a positive regulator of PACT-dependent PKR activation after RNA virus infections.PLoS Pathog. 2025 Jun 16;21(6):e1013246. doi: 10.1371/journal.ppat.1013246. eCollection 2025 Jun. PLoS Pathog. 2025. PMID: 40522981 Free PMC article.
-
Improvement of In Vivo Expression of Genes Delivered by Self-Amplifying RNA Using Vaccinia Virus Immune Evasion Proteins.Hum Gene Ther. 2017 Dec;28(12):1138-1146. doi: 10.1089/hum.2017.121. Epub 2017 Sep 6. Hum Gene Ther. 2017. PMID: 28877647 Free PMC article.
-
The Potential of Extracellular Vesicle-Mediated Spread of Self-Amplifying RNA and a Way to Mitigate It.Int J Mol Sci. 2025 May 26;26(11):5118. doi: 10.3390/ijms26115118. Int J Mol Sci. 2025. PMID: 40507927 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
The advent of clinical self-amplifying RNA vaccines.Mol Ther. 2025 Jun 4;33(6):2565-2582. doi: 10.1016/j.ymthe.2025.03.060. Epub 2025 Apr 3. Mol Ther. 2025. PMID: 40186353 Review.
References
-
- Ramos da Silva J, Bitencourt Rodrigues K, Formoso Pelegrin G, Silva Sales N, Muramatsu H, de Oliveira Silva M, Porchia B, Moreno ACR, Aps L, Venceslau‐Carvalho AA et al. (2023) Single immunizations of self‐amplifying or non‐replicating mRNA‐LNP vaccines control HPV‐associated tumors in mice. Sci Transl Med 15, eabn3464. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

