The feasibility of monitoring trauma patients with a wireless, wearable Doppler ultrasound
- PMID: 40200788
- PMCID: PMC12035993
- DOI: 10.1111/trf.18241
The feasibility of monitoring trauma patients with a wireless, wearable Doppler ultrasound
Abstract
Background: Early detection of reduced stroke volume (SV) or cardiac output (CO) may expedite resuscitative interventions during traumatic hemorrhage; corrected carotid artery flow time (ccFT) has been proposed as a surrogate for SV during blood volume loss.
Study design and methods: We conducted a prospective cohort study to assess the feasibility of using a wireless, wearable Doppler ultrasound capable of measuring ccFT in traumatically injured patients at a level 1 trauma center. A convenience sample of 33 patients was enrolled. We assessed device placement, data transfer and capture, and signal quality by assessing the ability to capture at least 15 consecutive cardiac cycles in the minute prior to blood pressure monitor cycling. A post hoc analysis examined ccFT variations between transfused and non-transfused patients.
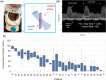
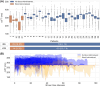
Results: Device placement succeeded in 94% of patients (n = 31) and the data were captured and transferred from all 31. The consecutive cardiac cycles before blood pressure measurement exceeded 15 (p = .015) in 93% of patients (n = 28). We observed ccFT below 270 ms and longer time spent under this threshold during resuscitation in transfused patients. Patients with low ccFT experienced more severe injuries and longer hospital and ICU stays.
Discussion: This is the first study that demonstrates the feasibility of using a wearable Doppler ultrasound in trauma patients on admission to the trauma bay. Although findings suggest that ccFT could serve as an early marker of hemodynamic compromise, further large-scale, multicenter studies are needed to validate its predictive value and clinical utility in guiding trauma resuscitation.
Keywords: Doppler ultrasound; RBC transfusion; blood management; corrected carotid flow time; transfusion practices (adult); wearable technology.
© 2025 The Author(s). Transfusion published by Wiley Periodicals LLC on behalf of AABB.
Conflict of interest statement
SA, DF, DJ, J‐ESK, JKE work for Flosonics Medical, the start‐up building the wearable Doppler ultrasound. LDL, LN, RI, and DP disclosed no conflicts of interest.
Figures
References
-
- O'Keeffe S, Paramalingam R, Grobler C. It's about bloody time!: the massive transfusion protocol in trauma. Aust Anaesth. 2019;2019:115–125.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

