Discordance in Claudin 18.2 Expression Between Primary and Metastatic Lesions in Patients With Gastric Cancer
- PMID: 40200874
- PMCID: PMC11982508
- DOI: 10.5230/jgc.2025.25.e2
Discordance in Claudin 18.2 Expression Between Primary and Metastatic Lesions in Patients With Gastric Cancer
Abstract
Purpose: Claudin 18.2 (CLDN18.2) has emerged as a promising therapeutic target for CLDN18.2-expressing gastric cancer (GC). We sought to examine the heterogeneity of CLDN18.2 expression between primary GC (PGC) and metastatic GC (MGC) using various scoring methods.
Materials and methods: We retrospectively analyzed data from 102 patients with pathologically confirmed paired primary and metastatic gastric or gastroesophageal junction adenocarcinomas. CLDN18.2 expression was evaluated through immunohistochemistry on formalin-fixed paraffin-embedded tissue samples. We assessed CLDN18.2 positivity using multiple scoring approaches, including the immunoreactivity score, H-score, and the percentage of tumor cells showing moderate-to-strong staining intensity. We analyzed the concordance rates between PGC and MGC and the association of CLDN18.2 positivity with clinicopathological features.
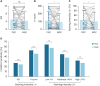
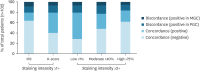
Results: CLDN18.2 positivity varied from 25% to 65% depending on the scoring method, with PGC consistently showing higher expression levels than MGC. Intratumoral heterogeneity was noted in 25.5% of PGCs and 19.6% of MGCs. Intertumoral heterogeneity, manifesting as discordance in CLDN18.2 positivity between PGC and MGC, was observed in about 20% of cases, with moderate agreement across scoring methods (κ=0.47 to 0.60). In PGC, higher CLDN18.2 positivity correlated with synchronous metastasis, presence of peritoneal metastasis, poorly differentiated grade, and biopsy specimens. In MGC, positivity was associated with synchronous metastasis, presence of peritoneal metastasis, and metastatic peritoneal tissues.
Conclusions: CLDN18.2 expression demonstrates significant heterogeneity between PGC and MGC, with a 20% discordance rate. Comprehensive tissue sampling and reassessment of CLDN18.2 status are crucial, especially before initiating CLDN18.2-targeted therapies.
Keywords: Claudin; Gastric cancer; Metastasis.
Copyright © 2025. Korean Gastric Cancer Association.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures



References
-
- Shah MA, Kennedy EB, Alarcon-Rozas AE, Alcindor T, Bartley AN, Malowany AB, et al. Immunotherapy and targeted therapy for advanced gastroesophageal cancer: ASCO guideline. J Clin Oncol. 2023;41:1470–1491. - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

