Peanut challenges prior to oral immunotherapy demonstrate high tolerance rates in selected patients
- PMID: 40201038
- PMCID: PMC11978367
- DOI: 10.1016/j.jacig.2025.100442
Peanut challenges prior to oral immunotherapy demonstrate high tolerance rates in selected patients
Abstract
Background: Peanut oral immunotherapy (pOIT) protocols typically remain below the threshold for reaction during the initial dose escalation (IDE) day. However, some patients may have higher thresholds for reaction or may not have an ongoing peanut allergy.
Objective: We sought to characterize the response to an accelerated initial dose escalation (A-IDE) for qualifying low-risk peanut-allergic patients younger than 4 years in which IDE progressed to a full peanut oral food challenge as tolerated.
Methods: Records of 76 pOIT patients younger than 4 years were reviewed. Those with history of peanut reaction with peanut allergy testing of less than 95% positive predictive value for failed oral food challenge were offered an A-IDE. A-IDE proceeded stepwise until patients refused dosing, any reaction occurred, or they tolerated the challenge (cumulative dose: 4000 mg peanut protein). If the A-IDE was not tolerated, patients completed pOIT.
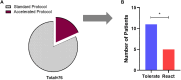
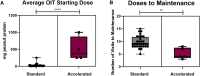
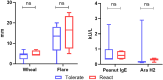
Results: From April 2022 to February 2024, 16 patients participated in an A-IDE. Eleven (68.8%) tolerated the 4000 mg cumulative dose, demonstrating resolution of their peanut allergy. The remaining had mild symptoms not requiring epinephrine. Mean pOIT starting dose following A-IDE was 450 mg (vs 25 mg in standard pOIT). Maintenance dosing was reached with a mean of 5.2 visits (vs 9.7 in standard pOIT).
Conclusions: Nearly 70% of low-risk patients younger than 4 years with previous diagnosis of peanut allergy tolerated a full peanut serving when initiating pOIT. This indicates the importance of diagnostic peanut challenge to selected patients before initiating OIT.
Keywords: Food allergy; oral food challenge; oral immunotherapy; peanut.
© 2025 The Author(s).
Conflict of interest statement
C.F.S., I.F.S, and K.M.O. receive grant support from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (award no. U01AI18182-01). C.F.S. also receives grant support from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (award no. K23AI162661) and the University of Michigan via the Ronald Koenig, MD, PhD, Department of Internal Medicine Early Career Endowment. Disclosure of potential conflict of interest: K. M. O’Shea, G. M. Sanders, and I. F. Slack receive grant support from Aimmune, DBV Technologies, Novartis Pharmaceuticals, Alladapt Immunotherapeutics, ALK-Abelló, and Siolta Therapeutics. The rest of the authors declare that they have no relevant conflicts of interest.
Figures
References
-
- Soller L., Abrams E.M., Carr S., Kapur S., Rex G.A., Leo S., et al. First real-world effectiveness analysis of preschool peanut oral immunotherapy. J Allergy Clin Immunol Pract. 2021;9:1349–1356.e1. - PubMed
-
- Nguyen K., Lewis M.O., Hanna E., Alfaro M.K.C., Corrigan K., Buonanno J., et al. Safety of multifood oral immunotherapy in children aged 1 to 18 years at an academic pediatric clinic. J Allergy Clin Immunol Pract. 2023;11:1907–1913.e1. - PubMed
-
- Blumchen K., Ulbricht H., Staden U., Dobberstein K., Beschorner J., de Oliveira L.C., et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126:83–91.e1. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

