Boosting Ru atomic efficiency of LaFe0.97Ru0.03O3 via knowledge-driven synthesis design
- PMID: 40201168
- PMCID: PMC11973924
- DOI: 10.1039/d5sc00778j
Boosting Ru atomic efficiency of LaFe0.97Ru0.03O3 via knowledge-driven synthesis design
Abstract
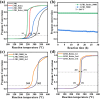
The exsolution of ruthenium from a 3 at% ruthenium-substituted LaFeO3 (LFR3) perovskite oxide is meticulously designed to produce a high-performance ruthenium-supported catalyst with high atomic efficiency. A high-temperature redox pretreatment at 800 °C enriches the Ru concentration in the near-surface region of LFR3, while a subsequent mild reduction step with H2 at 500 °C leads to the Ru exsolution from the Ru-enriched near-surface region (LFR3_Redox_500R), resulting in a high density of small particles that are not passivated by LaO x . The performance of this catalyst is evaluated through its application in two prototypical catalytic reactions: the combustion of propane (oxidation reaction) and the reduction of CO2 by hydrogenation (reduction reaction). For both reactions, the activity of the redox-pretreated sample LFR3_Redox_500R exhibits a significant increase compared to the activity of the untreated sample (LFR3_500R). In the catalytic hydrogenation of CO2, the high selectivity profile undergoes a transition from CO for LFR3_500R to methane for LFR3_Redox_500R.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- Kwon O. Joo S. Choi S. Sengodan S. Kim G. JPhys Energy. 2020;2:032001. doi: 10.1088/2515-7655/ab8c1f. - DOI
-
- Yang Y. Li J. Sun Y. Chem. Eng. J. 2022;440:135868. doi: 10.1016/j.cej.2022.135868. - DOI
-
- Zhang J. Gao M.-R. Luo J.-L. Chem. Mat. 2020;32:5424–5441.
-
- Sun Z. Hao C. Toan S. Zhang R. Li H. Wu Y. Liu H. Sun Z. J. Mater. Chem. A. 2023;11:17961–17976.
LinkOut - more resources
Full Text Sources

