Plasmodium falciparum reticulocyte-binding homologues are targets of human inhibitory antibodies and play a role in immune evasion
- PMID: 40201183
- PMCID: PMC11975925
- DOI: 10.3389/fimmu.2025.1532451
Plasmodium falciparum reticulocyte-binding homologues are targets of human inhibitory antibodies and play a role in immune evasion
Abstract
Introduction: Antibodies targeting the blood-stage of Plasmodium falciparum play a critical role in naturally acquired immunity to malaria by limiting blood-stage parasitemia. One mode of action of antibodies is the direct inhibition of merozoite invasion of erythrocytes through targeting invasion ligands. However, evasion of inhibitory antibodies may be mediated in P. falciparum by switching between various ligand-mediated merozoite invasion pathways. Here, we investigated the potential roles of invasion ligands PfRH1, PfRH2a and PfRH2b in immune evasion through phenotypic variation, and their importance as targets of human invasion-inhibitory antibodies.
Methods: Serum samples from malaria-exposed children and adults in Kenya were examined for their ability to inhibit P. falciparum invasion, using parasites with disrupted pfrh1, pfrh2a or pfrh2b genes.
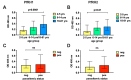
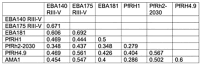
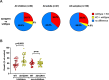
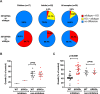
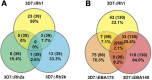
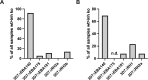
Results and discussion: The loss of PfRH1 and PfRH2b substantially impacted on susceptibility to inhibitory antibodies, suggesting that variation in the use of these ligands contributes to immune evasion. The effect was less prominent with loss of PfRH2a. Differential inhibition of the knockout and parental lines points to PfRH1 and PfRH2b as targets of acquired growth inhibitory antibodies whereas PfRH2a appeared to be a minor target. There was limited relatedness of the inhibitory responses between different isolates or compared to parasites with deletions of erythrocyte-binding antigens. This further suggests that there is a substantial amount of antigenic diversity in invasion pathways to facilitate immune evasion. These findings provide evidence that PfRH1 and PfRH2b are significant targets of inhibitory antibodies and variation in their expression may facilitate immune evasion. Targeting of multiple invasion ligands in vaccine design is likely to be required to achieve potent inhibitory antibodies and protective efficacy against malaria.
Keywords: P. falciparum; RH proteins; immune evasion; inhibitory antibodies; phenotypic variation; reticulocyte binding homologues.
Copyright © 2025 Reiling, Persson, McCallum, Gicheru, Kinyanjui, Chitnis, Fowkes, Marsh and Beeson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Aitken EH, Mbewe B, Luntamo M, Maleta K, Kulmala T, Friso MJ, et al. . Antibodies to chondroitin sulfate a-binding infected erythrocytes: dynamics and protection during pregnancy in women receiving intermittent preventive treatment. J Infect Dis. (2010) 201:1316–25. doi: 10.1086/651578 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

