Comparative evaluation of the effectiveness and acceptance of intranasal dexmedetomidine and intranasal midazolam for sedation in children aged 5-8 years using a mucosal atomizer device: a randomized controlled clinical study
- PMID: 40201232
- PMCID: PMC11972927
- DOI: 10.17245/jdapm.2025.25.2.109
Comparative evaluation of the effectiveness and acceptance of intranasal dexmedetomidine and intranasal midazolam for sedation in children aged 5-8 years using a mucosal atomizer device: a randomized controlled clinical study
Abstract
Background: Patient age, preoperative anxiety, dental requirement, risks associated with pharmaceutical management, safety, parental expectations, and cost influence the choice of pharmacological behavior management. Thus, this randomized controlled clinical study aimed to compare the effectiveness and acceptance of intranasal dexmedetomidine and midazolam for sedation in children aged 5-8 years using a mucosal atomizer device (MAD).
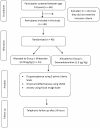
Methods: A total of 48 participants with Frankl's II behavior were randomly divided into two groups: Group I received intranasal midazolam (0.25 mg/kg), and Group II received intranasal dexmedetomidine (1.5 µg/kg). The primary outcomes assessed were drug acceptance, onset and effectiveness of sedation, and pre-and post-treatment anxiety levels. Secondary measures were also evaluated pre- and post-treatment.
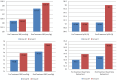
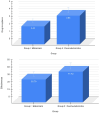
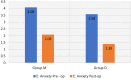
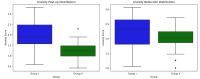
Results: Intranasal dexmedetomidine demonstrated significantly better drug acceptance (P < 0.001). Midazolam had a faster onset but was less effective than dexmedetomidine (P < 0.001). Additionally, dexmedetomidine exhibited better anxiolytic properties than midazolam (P < 0.001).
Conclusion: Dexmedetomidine was better accepted by children aged 5-8 years, was more effective, and had superior anxiolytic properties compared with midazolam.
Keywords: Administration, Intranasal; Anxiety; Dexmedetomidine; Drug Delivery Systems; Midazolam.
Copyright © 2025 Journal of Dental Anesthesia and Pain Medicine.
Conflict of interest statement
DECLARATION OF INTERESTS: The authors declare no conflict of interest related to this study. This study was conducted without external funding.
Figures
References
-
- American Academy of Pediatric Dentistry. Policy on the role of pediatric dentists as both primary and specialty care providers. The Reference Manual of Pediatric Dentistry. Chicago, III.: American Academy of Pediatric Dentistry; 2022. p. 171.
-
- Armfield JM. Cognitive vulnerability: a model of the etiology of fear. Clin Psychol Rev. 2006;26:746–768. - PubMed
-
- American Academy of Pediatric Dentistry. Behavior guidance for the pediatric dental patient. The Reference Manual of Pediatric Dentistry. Chicago, III.: American Academy of Pediatric Dentistry; 2022. pp. 321–339.
-
- Klingberg G, Broberg AG. Dental fear/anxiety and dental behaviour management problems in children and adolescents: a review of prevalence and concomitant psychological factors. Int J Paediatr Dent. 2007;17:391–406. - PubMed
-
- Wilson S, Alcaino EA. Survey on sedation in paediatric dentistry: a global perspective. Int J Paediatr Dent. 2011;21:321–332. - PubMed
LinkOut - more resources
Full Text Sources

