Surgical technique of an innovative patient-specific metal implant for talar osteochondral lesions
- PMID: 40201356
- PMCID: PMC11976025
- DOI: 10.1002/jeo2.70085
Surgical technique of an innovative patient-specific metal implant for talar osteochondral lesions
Abstract
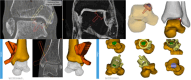
Purpose: Treatment strategies for osteochondral defects (OCDs) of the ankle have substantially increased over the last decade. The development of a small metallic implant to fill the defect has led to the second-generation patient-specific metal implant (Episealer Talus® Implant) designed based on computed tomography and magnetic resonance imaging images.
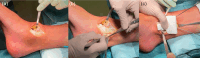
Methods: There is a pool of patients falling into the so-called 'treatment gap', a grey zone composed of active patients with symptomatic OCDs in the context of an otherwise healthy joint, or patients with a failed primary treatment. To minimize the risk of perioperative complications, there are a series of tips and tricks that can be considered.
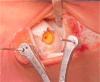
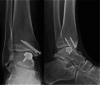
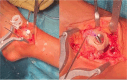
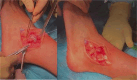
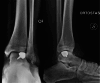
Results: Correct execution of the operative approach, proper positioning of the guides, posterior capsule and deep deltoid ligament release and the use of Hintermann spreader allow a perfect visualization of the OCDs minimizing the risk of iatrogenic lesions. Correct execution of the medial malleolus osteotomy, release of soft tissue, proper triplanar alignment of the custom-made guide, its strong stabilization during the reaming and the use of vigorous washes minimizes the potential damage on healthy cartilage. Correct sinking of the implant is crucial; the goal is to place the Episealer Talus at least 0.5 mm below the cartilage surface. Filling a large subchondral cyst with the cancellous bone can be useful to provide better stability of the implant.
Conclusion: Episealer Talus for talar OCDs possibly represents an additional tool for surgeons and patients. It is important to avoid mistakes during implant placement.
Levels of evidence: Level V, expert opinion.
Keywords: ankle; custom‐made implant; osteochondral lesions.
© 2025 The Author(s). Journal of Experimental Orthopaedics published by John Wiley & Sons Ltd on behalf of European Society of Sports Traumatology, Knee Surgery and Arthroscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Aurich, M. , Albrecht, D. , Angele, P. , Becher, C. , Fickert, S. , Fritz, J. et al. (2017) Treatment of osteochondral lesions in the ankle: a guideline from the group "clinical tissue regeneration" of the German Society of Orthopaedics and Traumatology (DGOU). Zeitschrift fur Orthopadie und Unfallchirurgie, 155(1), 92–99. Available from: 10.1055/s-0042-116330 - DOI - PubMed
-
- van Bergen, C.J.A. , Zengerink, M. , Blankevoort, L. , van Sterkenburg, M.N. , van Oldenrijk, J. & van Dijk, C.N. (2012) Novel metal implantation technique for secondary osteochondral defects of the medial talar dome – one‐year results of a prospective study. Fuß & Sprunggelenk, 10, 130–137. Available from: 10.1016/j.fuspru.2012.03.004 - DOI
-
- Dahmen, J. , Lambers, K.T.A. , Reilingh, M.L. , van Bergen, C.J.A. , Stufkens, S.A.S. & Kerkhoffs, G.M.M.J. (2018) No superior treatment for primary osteochondral defects of the talus. Knee surgery, sports traumatology, arthroscopy. Official Journal of the ESSKA, 26(7), 2142–2157. Available from: 10.1007/s00167-017-4616-5 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

