The effect of COVID vaccination timing on the seroprevalence of IgG antibodies: evidence from the Guayas region of Ecuador
- PMID: 40201360
- PMCID: PMC11975873
- DOI: 10.3389/fpubh.2025.1537049
The effect of COVID vaccination timing on the seroprevalence of IgG antibodies: evidence from the Guayas region of Ecuador
Abstract
Background and aims: Timely distribution of COVID-19 vaccines was particularly important for developing countries that do not have strong health systems and related infrastructure. We analyze data from the Guayas province of Ecuador, an area particularly affected by the pandemic, to determine the seroprevalence of SARS-CoV-2 and the effect of the timing of the second dose of COVID-19 vaccines on the seroprevalence SARS-CoV-2 IgG antibodies.
Methods: This cross-sectional study involved 1,761 individuals aged 18 and older who voluntarily enrolled prior to and during the initial phase of vaccine rollout in Ecuador (October 2020 to July 2022). IgG anti-SARS-CoV-2 RBD antibodies were assessed by an in-house ELISA to evaluate the immune response to Pfizer (BioNTech, Spike mRNA) and AstraZeneca (Oxford, AstraZeneca Spike) vaccine in the Guayas province. Ordinary least squares (OLS) regressions were employed to determine the effect of delayed second doses later than prescribed by the manufacturer for both vaccines.
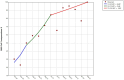
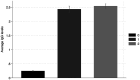
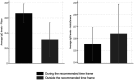
Results: Before the vaccination campaign, we estimated an RBD IgG seroprevalence of 27.7% (95% CI: 23.6-27, n = 469). The estimate increased to 89.4% (95% CI: 87.7-91.18, n = 1,235) after the first vaccine dose and to 92.6% (95% CI: 90.7-94.5, n = 748) after the second dose. Individuals who received the second dose of the Pfizer vaccine later than the recommended dose showed significantly lower levels of IgG antibodies 2-3 weeks after receiving the second dose than those who received the dose within the recommended timeframe. Furthermore, we did not find any effect on RBD IgG antibody levels in those who received a second dose of the AstraZeneca vaccine during the first and second parts of the recommended vaccination window.
Conclusion: The results suggest that a significant portion of the study population was already infected with SARS-CoV-2 prior to the vaccination. As expected, seropositivity increased alongside vaccination efforts. We determined that Pfizer vaccine recipients should be adhered to vaccine timing guidelines. Furthermore, resource-limited countries should consider administering vaccines with flexibility in dosing intervals, such as AstraZeneca, as it allows for a wider time frame without significantly reducing the boosting of IgG antibodies.
Keywords: COVID-19; IgG; SARS-CoV-2 RBD; cross-sectional study; in-house ELISA assay; seroprevalence; vaccine.
Copyright © 2025 Malacatus-Arboleda, Barbotó-Ramírez, Sánchez, Moscoso, Rhodes, Coloma, Guevara, Espinoza-Fuentes, Fernández-Cadena, Morey-León and Andrade-Molina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures
Similar articles
-
Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID-19 vaccines in Chile: a sentinel surveillance study.Lancet Infect Dis. 2022 Jan;22(1):56-63. doi: 10.1016/S1473-3099(21)00479-5. Epub 2021 Sep 9. Lancet Infect Dis. 2022. PMID: 34509185 Free PMC article.
-
Quantitative SARS-CoV-2 anti-spike responses to Pfizer-BioNTech and Oxford-AstraZeneca vaccines by previous infection status.Clin Microbiol Infect. 2021 Oct;27(10):1516.e7-1516.e14. doi: 10.1016/j.cmi.2021.05.041. Epub 2021 Jun 7. Clin Microbiol Infect. 2021. PMID: 34111577 Free PMC article.
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
Systematic review of seroprevalence of SARS-CoV-2 antibodies and appraisal of evidence, prior to the widespread introduction of vaccine programmes in the WHO European Region, January-December 2020.BMJ Open. 2023 Nov 6;13(11):e064240. doi: 10.1136/bmjopen-2022-064240. BMJ Open. 2023. PMID: 37931969 Free PMC article.
-
SARS-CoV-2 Vaccine-Induced Humoral Immunity in Immunocompetent European Adults: A Systematic Review.Microorganisms. 2025 Feb 27;13(3):535. doi: 10.3390/microorganisms13030535. Microorganisms. 2025. PMID: 40142428 Free PMC article. Review.
References
-
- Monge S, Rojas-Benedicto A, Olmedo C, Martín-Merino E, Mazagatos C, Limia A, et al. . Effectiveness of a second dose of an mRNA vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) omicron infection in individuals previously infected by other variants. Clin Infect Dis. (2023) 76:e367–74. doi: 10.1093/cid/ciac429, PMID: - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

