The impact of elevated temperature and salinity on microbial communities and food selectivity in heterotrophic nanoflagellates in the Boye River
- PMID: 40201423
- PMCID: PMC11976726
- DOI: 10.1093/ismeco/ycaf049
The impact of elevated temperature and salinity on microbial communities and food selectivity in heterotrophic nanoflagellates in the Boye River
Abstract
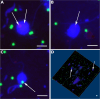
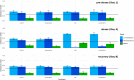
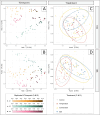
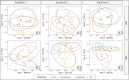
Microbial predator-prey interactions play a crucial role in aquatic food webs. Bacterivorous protists not only regulate the quantity and biomass of bacterial populations but also profoundly influence the structure of bacterial communities. Consequently, alterations in both the quantity and quality of protist bacterivory can influence the overall structure of aquatic food webs. While it is well-documented that changes in environmental conditions or the occurrence of abiotic stressors can lead to shifts in microbial community compositions, the impact of such disturbances on food selection remains unknown. Here, we investigated the effects of elevated temperature and salinization on food selectivity of heterotrophic nanoflagellates by monitoring the uptake of preselected target bacteria via catalyzed reporter deposition fluorescence in situ hybridization and fluorescence microscopy. Our results indicate that salinization, but not increased temperature, significantly increased the flagellates' selection against Microbacterium lacusdiani (Actinomycetota). However, the effect of the reduced grazing pressure was counterbalanced by the negative effect of increased salinity on the growth of Actinomycetota. Our results suggest that the effect of stressors on the feeding behavior of protistan predators may strongly affect the composition of their prey community, when bacterial taxa are concerned that are less sensitive to the particular stressor.
Keywords: CARD-FISH; amplicon sequencing; bacterivory; food web; freshwater ecology; heat stress; microbial communities; microscopy; predator–prey interactions; salinization.
© The Author(s) 2025. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
None declared.
Figures

References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

