In vitro Generated Megakaryocytes for the Detection of Human Platelet Antigen-Specific Alloantibodies
- PMID: 40201624
- PMCID: PMC11975340
- DOI: 10.1159/000539617
In vitro Generated Megakaryocytes for the Detection of Human Platelet Antigen-Specific Alloantibodies
Abstract
Introduction: Serologic characterization of antihuman platelet antigen (HPA) alloantibodies is crucial in fetal neonatal alloimmune thrombocytopenia. The gold standard MAIPA assay requires fresh platelets from HPA-genotyped donors, which is challenging for some laboratories. Megakaryocytes express HPA epitopes and offer an alternative source for detecting anti-HPA antibodies. The objective of this study was to assess the efficacy of a novel assay called monoclonal antibody immobilization of megakaryocyte antigens (MAIMA) for detecting anti-HPA antibodies.
Methods: CD34+ cells from buffy coats were differentiated into megakaryocytes in vitro. The performance of the MAIMA assay was evaluated using WHO reference reagents for HPA-1a, HPA-3a, and HPA-5b, along with sera samples from patients who had well-characterized anti-HPA antibodies.
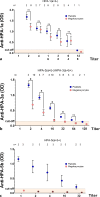
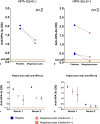
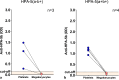
Results: The WHO anti-HPA-1a reference reagent showed similar binding to megakaryocytes and platelets in MAIMA and MAIPA, respectively. On the other hand, optical density (OD) values for the WHO anti-HPA-3a reference reagent were lower in MAIMA than in MAIPA. Anti-HPA-5b antibodies were not detectable in MAIMA. Patients' sera containing anti-HPA-1a antibodies were successfully detected in MAIMA in all clinical samples. Moreover, OD values in MAIPA and MAIMA showed high correlation (r = 0.96, p < 0.001). MAIMA was reactive for samples with anti-HPA-3a as well as anti-HPA-3b; however, OD values were lower compared to MAIPA. Interestingly, all patient samples with anti-HPA-5b antibodies were tested negative in MAIMA.
Conclusion: In vitro generated megakaryocytes can be used to detect anti-HPA-1a alloantibodies. However, despite this potential, they may be less suitable for the detection of alloantibodies against other HPAs such as HPA-5b.
Keywords: Antibodies; Buffy coat; Fetal alloimmune thrombocytopenia; Platelet antigens.
Plain language summary
The identification of antibodies to human platelet antigens (HPA) is crucial. The standard test (MAIPA assay) is difficult because it requires fresh platelets from specific donors. This study investigated a new method, monoclonal antibody immobilization of megakaryocyte antigens (MAIMA), using in vitro generated megakaryocytes from CD34+ cells. The assay was tested against WHO reference reagents and patient samples with known anti-HPA antibodies. Results showed that MAIMA successfully detected anti-HPA-1a antibodies and correlated well with MAIPA. However, MAIMA had lower sensitivity for anti-HPA-3a and anti-HPA-5b antibodies. Notably, anti-HPA-5b antibodies were not detectable by MAIMA. In conclusion, in vitro generated megakaryocytes can effectively detect anti-HPA-1a antibodies. However, MAIMA may be less suitable for antibodies against other HPAs, such as HPA-5b. This study highlights the potential of MAIMA as an alternative assay and provides insight into its strengths and limitations in detecting specific anti-HPA antibodies.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Tamam Bakchoul has received research funding from Stiftung Transfusionsmedizin und Immunhämatologie e.V. Other authors declare no competing financial interests.
Figures


Similar articles
-
Development of quantitative monoclonal antibody-specific immobilization of platelet antigens assay for antibodies against human platelet antigen-1a, 3a, and 5b.Platelets. 2018 Jan;29(1):71-75. doi: 10.1080/09537104.2017.1294681. Epub 2017 Apr 13. Platelets. 2018. PMID: 28406722
-
Evaluation of an enzyme-linked immunosorbent assay kit (GTI PakPlus) for the detection of antibodies against human platelet antigens.Transfus Med. 1999 Mar;9(1):63-7. doi: 10.1046/j.1365-3148.1999.009001063.x. Transfus Med. 1999. PMID: 10216906
-
Sensitive detection of platelet-specific antibodies with a modified MAIPA using biotinylated antibodies and streptavidin-coated beads.J Immunol Methods. 2016 Jul;434:9-15. doi: 10.1016/j.jim.2016.04.001. Epub 2016 Apr 5. J Immunol Methods. 2016. PMID: 27059653
-
Maternal HPA-1a antibody level and its role in predicting the severity of Fetal/Neonatal Alloimmune Thrombocytopenia: a systematic review.Vox Sang. 2019 Jan;114(1):79-94. doi: 10.1111/vox.12725. Epub 2018 Nov 22. Vox Sang. 2019. PMID: 30565711
-
Anti-human platelet antigen-5b antibodies and fetal and neonatal alloimmune thrombocytopenia; incidental association or cause and effect?Br J Haematol. 2022 Jul;198(1):14-23. doi: 10.1111/bjh.18173. Epub 2022 Apr 5. Br J Haematol. 2022. PMID: 35383895 Review.
References
-
- Bussel JB, Vander Haar EL, Berkowitz RL. New developments in fetal and neonatal alloimmune thrombocytopenia. Am J Obstet Gynecol. 2021;225(2):120–7. - PubMed
-
- Kovanlikaya A, Tiwari P, Bussel JB. Imaging and management of fetuses and neonates with alloimmune thrombocytopenia. Pediatr Blood Cancer. 2017;64(12). - PubMed
-
- de Vos TW, Winkelhorst D, Árnadóttir V, van der Bom JG, Canals Surís C, Caram-Deelder C, et al. . Postnatal treatment for children with fetal and neonatal alloimmune thrombocytopenia: a multicentre, retrospective, cohort study. Lancet Haematol. 2022;9(11):e844–53. - PubMed
-
- Kamphuis MM, Paridaans N, Porcelijn L, De Haas M, van der Schoot CE, Brand A, et al. . Screening in pregnancy for fetal or neonatal alloimmune thrombocytopenia: systematic review. BJOG. 2010;117(11):1335–43. - PubMed
-
- Versiti: human platelet antigen (HPA) database. Versiti. https://www.versiti.org/products-services/human-platelet-antigen-hpa-dat... (accessed January 19, 2023).
LinkOut - more resources
Full Text Sources

