Prognostic value of baseline EORTC QLQ-C30 scores for overall survival across 46 clinical trials covering 17 cancer types: a validation study
- PMID: 40201799
- PMCID: PMC11976232
- DOI: 10.1016/j.eclinm.2025.103153
Prognostic value of baseline EORTC QLQ-C30 scores for overall survival across 46 clinical trials covering 17 cancer types: a validation study
Abstract
Background: A pooled data analysis by Quinten et al. (2009) found three European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) health-related quality of life (HRQoL) scales to be prognostic for survival: physical functioning, pain and appetite loss. This study aims to replicate these findings in an independent data set comprising a broader cancer population.
Methods: Data were obtained from 46 clinical trials across three cancer research networks conducted between 1996 and 2013 that assessed HRQoL using the EORTC QLQ-C30. A stratified Cox proportional hazards model was employed to assess the prognostic significance of baseline QLQ-C30 scale scores on overall survival, adjusting for socio-demographic and clinical variables. Stepwise model selection was done at 5% significance level. Model stability and prognostic accuracy were evaluated via bootstrapping and the C index respectively.
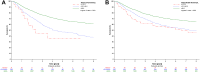
Findings: Data from 16,210 patients reporting HRQoL at baseline, spanning 17 cancer types, was used. The stratified multivariable model confirmed that better physical functioning (hazard ratio [HR], 0.94; 95% confidence interval [CI], 0.93-0.96), lower pain (HR, 1.02; 95% CI, 1.01-1.03), and appetite loss (HR, 1.04; 95% CI, 1.03-1.05) were significantly associated with survival. Additionally, global health status/QoL, dyspnoea, emotional and cognitive functioning were found to be prognostic for survival. This final model, encompassing sociodemographic, clinical, and HRQoL variables, achieved a corrected C index of 0.74, marking a 48% enhancement in discriminatory ability. Bootstrap evaluation indicated no major instability issues.
Interpretation: These results support previous findings that baseline physical functioning, pain, and appetite loss scores, along with four other scales from the EORTC QLQ-C30, predict survival in cancer patients.
Funding: EORTC Quality of Life Group.
Keywords: Cancer clinical trial; Health-related quality of life; Pooled data; Prognostic factor analysis; Survival; Validation.
© 2025 The Authors.
Conflict of interest statement
E. Basch has received payments as a scientific advisor for Navigating Cancer, AstraZeneca, Resilience, and Verily. A Eggermont receives honoraria from BMS, Merck, and MSD. F. Cardoso has received payments for consulting fees from Amgen, Astellas/Medivation, AstraZeneca, Celgene, Daiichi-Sankyo, Eisai, GE Oncology, Genentech, Gilead, GlaxoSmithKline, Iqvia, Macrogenics, Medscape, Merck-Sharp, Merus BV, Mylan, Mundipharma, Novartis, Pfizer, Pierre-Fabre, prIME Oncology, Roche, Sanofi, Samsung Bioepis, Seagen, Teva, and Touchime. W.T.A. van der Graaf received an institutional grant from Eli Lilly, participated on an advisory board for Agenus and PTC Therapeutics, and is the President of EORTC and an European Cancer Organization board member. J.A.F. Koekkoek received payments for consulting fees from Fagron and grants paid to the institution from the EORTC Quality of Life Group, EORTC Brain Tumour Group, ZonMw, Team Westland, and the KWF Dutch Cancer Society. G. Velikova received payment from the University of Leeds for her work on this research; grants paid to the institution from the NIHR programme grant, Pfizer, and Yorkshire Cancer Research; payments for consulting fees from Pfizer, Roche, and Seagen; honoraria from Pfizer, Roche, Novartis, Eisai, and Sanofi; support for attending meetings from Pfizer and Roche; participated in a Data Safety Monitoring Board or Advisory Board for Roche, Seagen, and AstraZeneca; and has a leadership or fiduciary role on the EORTC Board of Directors and the NCRI Living with and beyond cancer group. A. Bottomley owns an independent private QOL consulting company that has clients in the pharmaceutical industry. All other authors declare no competing interests.
Figures
References
-
- Bottomley A., Flechtner H., Efficace F., et al. Health related quality of life outcomes in cancer clinical trials. Eur J Cancer. 2005;41:1697–1709. - PubMed
-
- Kluetz P.G., O’Connor D.J., Soltys K. Incorporating the patient experience into regulatory decision making in the USA, Europe, and Canada. Lancet Oncol. 2018;19:e267–e274. - PubMed
-
- US Food and Drug Administration . US Food and Drug Administration; Silver Spring, MD: 2009. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labelling claims.https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf
-
- European Medicines Agency . The evaluation of medicinal products, doc. Ref. EMEA/CHMP/EWP/139391/2004. European Medicines Agency, Committee For Medicinal Products For Human Use; London: 2005. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures.https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-p...
LinkOut - more resources
Full Text Sources

