The mitigating effect of dietary β-glucan against fipronil-induced intoxication in Nile Tilapia (Oreochromis niloticus): Histopathological, immunological, hematological, and biochemical analysis
- PMID: 40201840
- PMCID: PMC11974277
- DOI: 10.5455/OVJ.2025.v15.i2.45
The mitigating effect of dietary β-glucan against fipronil-induced intoxication in Nile Tilapia (Oreochromis niloticus): Histopathological, immunological, hematological, and biochemical analysis
Abstract
Background: Due to its remarkable effectiveness against a wide range of pests and insects at very low concentrations, the broad-spectrum fipronil pesticide is currently gaining popularity in the agricultural, public health, and international industries. However, the stressor effects of fipronil insecticides cause ecological disruption, growth retardation, immunosuppression, and higher fish mortality rates. Both animals and humans have demonstrated the effectiveness of prebiotics such as β-1,3-glucan in their diets. Aquaculture has recently increased in use because of its potential to control diseases, compete with environmental stresses, and promote fish growth.
Aim: The goal of this study was to determine how dietary β-1,3-glucan can protect Nile Tilapia (Oreochromis niloticus)fish from fipronil's harmful effects.
Methods: We randomly divided 240 fish into four equal groups. As a control, the first group (G1) was fed a standard diet. A 0.1% dose of -1, 3-glucan was added to G2. Fipronil was added to G3 at a concentration of 2.8 mg/l (1/10 96 h LC50). At the indicated concentrations, G4 was combined with β-1, 3-glucan, and fipronil. Alterations in vital signs, metabolic profiles, immunological responses, blood counts, and any histological abnormalities in the liver or spleen of the fish were investigated and recorded.
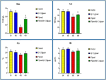
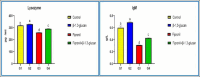
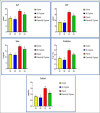
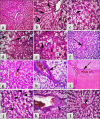
Results: The fipronil-exposed group exhibited slow mobility, respiratory discomfort, and increased mucus secretion. Several blood markers, like immunoglobulin M and lysozyme, were found to be significantly lower. On the other hand, the levels of aspartate aminotransferase, alanine aminotransferase, urea, creatinine, and cortisol in the serum were significantly higher. Liver histopathology revealed hemorrhagic blood vessels, steatosis in hepatocytes, hydropic degeneration, and widespread necrosis. Furthermore, we noted serious splenic parenchymal necrosis, hemorrhagic red pulp, white pulp depletion, and hemosiderosis. Histological changes were slowed by G4, which had β-1,3-glucan and fipronil. Moreover, it increases blood markers and physical activity levels.
Conclusion: The results show that β-1,3-glucan is an effective dietary supplement for Nile tilapia, and it improves their health, increases their immunity, and neutralizes fipronil contaminants in fish farming.
Keywords: Biochemical; Fipronil; Histopathological; Immunological; Nile Tilapia; Toxicity; β-1,3-glucan.
Conflict of interest statement
No competing interests.
Figures

References
-
- Abouelghar G.E., El-Bermawy Z.A., Salman H.M.S. Oxidative stress, hematological and biochemical alterations induced by sub-acute exposure to fipronil (COACH®) in albino mice and ameliorative effect of selenium plus vitamin E. Environ. Sci. Pollut. Res. Int. 2020;27(8):7886–900. - PubMed
-
- Ansoar-Rodríguez Y., Christofoletti C.A., Correia J.E., de Souza R.B., Moreira-de-Sousa C., Marcato A.C., Bueno O.C., Malaspina O., Silva-Zacarin E.C., Fontanetti C.S. Liver alterations in Oreochromis niloticus (Pisces) induced by insecticide imidacloprid: histopathology and heat shock protein in situ localization. J. Environ. Sci. Health B. 2016;51(12):881–7. - PubMed
-
- Ansoar-Rodrıguez Y., Christofoletti C.A., Marcato A.C., Correia J.E., Bueno O.C., Malaspina O., Fontanetti C.S. Genotoxic potential of the insecticide imidacloprid in a non-target organism (Oreochromis niloticus-Pisces) J. Environ. Prot. 2015;6(12):1360–7.
-
- AnvariFar H., Amirkolaie A.K., Jalali A.M., Miandare H.K., Sayed A.H., Üçüncü S.İ., Ouraji H., Ceci M., Romano N. Environmental pollution and toxic substances: cellular apoptosis as a key parameter in a sensible model like fish. Aquat. Toxicol. 2018;204:144–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
