Antiviral potential and stability analysis of chicken interferon-α produced by Newcastle disease virus in chicken embryo fibroblast cells
- PMID: 40201855
- PMCID: PMC11975359
- DOI: 10.17221/106/2020-VETMED
Antiviral potential and stability analysis of chicken interferon-α produced by Newcastle disease virus in chicken embryo fibroblast cells
Abstract
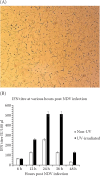
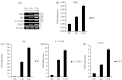
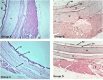
Chicken interferon-α (chIFN-α) is an important antiviral cytokine and represents one of the first lines of the chicken's innate immune system. The current study is the first-ever report of chicken IFN (chIFN) production in Pakistan. In this study, we have used live and UV-irradiated Newcastle disease virus (NDV) to induce the expression of chIFN-α in chicken embryo fibroblast (CEF) cells. ChIFN-α was partially purified in a two-step protocol; ultracentrifugation followed by treatment with anti-chIFN-β antibodies. The purified chIFN-α was ana-lysed via sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and the in vitro antiviral potential of chIFN-α was determined against the H9N2 avian influenza virus (AIV) via a cytopathic inhibition assay. The relative mRNA level of the IFN-stimulated genes (ISGs) in the IFN-stimulated CEF cells was measured at various time intervals by a quantitative polymerase chain reaction (qPCR). The stability of natural chIFN-α to the temperature, pH, and ultraviolet (UV) light was also determined. The in vivo therapeutic potential of chIFN-α was determined in 7-day-old broiler chickens challenged with AIV. We found that a higher chIFN-α expression level was induced by the UV-irradiated NDV in the CEF cells as compared to the live NDV. The UV-irradiated NDV induced the maximum IFN production in the CEF cells at 24 h post-infection. Two bands of 21 kDa on SDS-PAGE confirmed the presence of the chIFN-α protein. The cytopathic inhibition assay indicated the strong antiviral activity of chIFN-α against AIV. Our results of the stability analysis showed that chIFN-α was stable at a wide range of temperatures and pH levels. However, a little exposure to UV-light resulted in a significant loss of antiviral activity. We also observed that the antiviral activity of chIFN-α is related to the expression levels of the antiviral ISGs. The results of the in vivo study showed that the chIFN-α therapy via the oral route resulted in a significant improvement in the tracheal pathology of chickens challenged with AIV. In conclusion, we suggest that chIFN-α could be an important therapeutic tool to control avian influenza infection in poultry.
Keywords: IFN-stimulated genes; Newcastle disease virus; antiviral; chicken embryo fibroblast; chicken type I IFNs; cytopathic inhibition assay; innate immunity.
Copyright: © 2021 CAAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Anjum FR, Rahman SU, Aslam MA, Qureshi AS. Comprehensive network map of transcriptional activation of chicken type I IFNs and IFN-stimulated genes. Comp Immunol Microbiol Infect Dis. 2020a Dec;68:101407. - PubMed
-
- Anjum FR, Anam S, Rahman SU, Ali S, Aslam MA, Rizvi F, Asif M, Abdullah RM, Abaidullah M, Shakir MZ, Goraya MU. Anti-chicken type I IFN countermeasures by major avian RNA viruses. Virus Res. 2020b Sep;16:198061. - PubMed
-
- Daviet S, Van Borm S, Habyarimana A, Ahanda ML, Morin V, Oudin A, Van Den Berg T, Zoorob R. Induction of Mx and PKR failed to protect chickens from H5N1 infection. Viral Immunol. 2009 Dec;22(6):467-72. - PubMed
-
- Ellis MN, Eidson CS, Brown J, Kleven SH. Studies on interferon induction and interferon sensitivity of avian reoviruses. Avian Dis. 1983 Oct-Dec;27(4):927-36. - PubMed
LinkOut - more resources
Full Text Sources
