Systematic Optimization of the Synthesis of Confined Carbyne
- PMID: 40201933
- PMCID: PMC12391636
- DOI: 10.1002/smtd.202500075
Systematic Optimization of the Synthesis of Confined Carbyne
Abstract
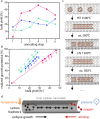
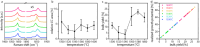
Confined carbyne, an sp1-hybridized linear carbon chain inside a carbon nanotube, is a novel material with remarkable properties and potential applications. Among its currently successful synthesis methods, high temperature high vacuum annealing is prevalent. Further optimization could be achieved by tuning the synthesis pathway. Here, a systematic analysis of key synthesis parameters including precursor filling, annealing step sequences, and temperature conditions during high temperature vacuum processing is performed. A novel yield determination model that overcomes previous limitations related to the irregular resonance Raman behavior of carbyne is applied to evaluate bulk yield and realized growth potential. With this refined model, it is possible to make a quantitative assessment of bulk yield optimization potential in multi-step annealing processes. These results provide crucial insights into the fundamental formation mechanisms of confined carbyne, advancing our understanding of this promising hybrid nanomaterial system. It is therefore possible to establish improved protocols for maximizing confined carbyne yields through precise control of synthesis conditions.
Keywords: Raman spectroscopy; carbon nanotubes; carbyne; confined carbyne; synthesis.
© 2025 The Author(s). Small Methods published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Al‐Backri A., Zólyomi V., Lambert C. J., J. Chem. Phys. 2014, 140, 10. - PubMed
-
- Cui Y., Wei Q., Park H., Lieber C. M., science 2001, 293, 1289. - PubMed
-
- Fazio E., Neri F., Patanè S., D'urso L., Compagnini G., Carbon 2011, 49, 306.
-
- Liu M., Artyukhov V. I., Lee H., Xu F., Yakobson B. I., ACS nano 2013, 7, 10075. - PubMed
-
- Wang Y., Huang Y., Yang B., Liu R., Carbon 2006, 44, 456.
Grants and funding
LinkOut - more resources
Full Text Sources

