The #HOPE4LIVER Single-arm Pivotal Trial for Histotripsy of Primary and Metastatic Liver Tumors: One-year Update of Clinical Outcomes
- PMID: 40201962
- PMCID: PMC12594125
- DOI: 10.1097/SLA.0000000000006720
The #HOPE4LIVER Single-arm Pivotal Trial for Histotripsy of Primary and Metastatic Liver Tumors: One-year Update of Clinical Outcomes
Abstract
Objective: To evaluate the 1-year clinical outcomes of patients enrolled in the #HOPE4LIVER trial of hepatic histotripsy.
Background: Histotripsy is a novel noninvasive, non-thermal-focused ultrasound therapy that liquefies tissue at the focal point of the transducer. Following diagnostic ultrasound targeting, an automated treatment is performed through a robotic arm to treat a user-defined volume of tissue.
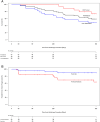
Methods: Forty-seven patients were enrolled at 14 sites in the United States and Europe. Included patients were ineligible for or had opted out of standard therapies. Tumor control was evaluated through a core laboratory with a primary assessment at each time point and a post hoc assessment performed following the completion of each time point to allow for a learning curve of interpreting imaging findings of this novel therapy. Overall survival and freedom from local tumor progression were evaluated through the Kaplan-Meier method.
Results: Nineteen patients with hepatocellular carcinoma and 28 with metastatic disease were enrolled, of whom 89.5% (17/19) and 96.4% (27/28) had multifocal hepatic tumors at the time of treatment. Fifty-two tumors were treated. The 1-year local control rate was 63.4% using the primary assessment method and 90% using the post hoc method. There were 6 serious adverse device-related effects within 30 days of treatment. Only one nonserious adverse device-related effect was observed after 30 days of treatment. Overall survival at 1-year was 73.3% for patients with hepatocellular carcinoma and 48.6% for patients with metastatic disease.
Conclusions: Histotripsy results in local control of liver tumors at 1 year, which is consistent with current locoregional therapies. The safety profile is favorable, and survival at 1 year is comparable with other therapies for similar disease stages.
Keywords: ablation; hepatic metastasis; hepatocellular carcinoma; histotripsy.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
T.J.Z. Grants, HistoSonics, Ethicon, National Cancer Institute of National Institutes of Health (RO1CA262474); consulting fees from Elephas and Histosonics; Participation in a Data Safety Monitoring Board or Advisory Board from Elephas; stock or stock options from HistoSonics, equipment provided to institution from HistoSonics; M.M.L. Support for attending meetings from HistoSonics; ACR and SAR LI-RADS Treatment Response Working Group chair. T.M.W. Grants from Boston Scientific, Angiodynamics, HistoSonics, Johnson and Johnson; consulting fees from Angiodynamics; payment for lectures from Boston Scientific, Angiodynamics, Johnson and Johnson; participation in a Data Safety Monitoring Board or Advisory Board from Angiodynamics, Johnson and Johnson; leadership or fiduciary roles from Faculty Board Royal College of Radiologists, British Society of Interventional Radiology, NIHR, NICE. G.N. Consulting fees from Angiodynamics, Boston Scientific, Varian Interventional Solutions, Stryker, Philips; scientific advisory board for Quantum Surgical and Beta Glue. S.B.W. Consulting fees from Guerbet, Cook Medical, AstraZeneca; payment for lectures from Penumbra; participation on a Data Safety Monitoring Board or Advisory Board from AstraZeneca, Merck, Trisalus, Esai; Member of the SIO board of directors; deputy editor for Radiology. O.A. Grants from Canon Medical; consulting fees from Boston Scientific, Angiodynamics, Johnson and Johnson, Asahi Medical, Argon Medical, Bard; payment for lectures from Penumbra; chief medical officer and equity owner of Flow Medical. N.D.P. Grants or contracts from Exelixis, Genentech, Bayer, TARGET, National Institutes of Health; consulting fees from AstraZeneca, Genentech, Esai, Gilead. C.S.C. Consulting fees for participation as a co-principal investigator for HistoSonics; patent holder for use of histotripsy to generate immunogenic tumor vaccines. The remaining authors report no conflicts of interest.
Figures
References
-
- Ceppa EP, Collings AT, Abdalla M, et al. SAGES/AHPBA guidelines for the use of microwave and radiofrequency liver ablation for the surgical treatment of hepatocellular carcinoma or colorectal liver metastases less than 5 cm. Surg Endosc. 2023;37:8991–9000. - PubMed
-
- Cervantes A, Adam R, Roselló S, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10–32. - PubMed
-
- National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Colon Cancer (Version 5.2024). Accessed October 11, 2024. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1428
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

