Nanoscale warriors against viral invaders: a comprehensive review of Nanobodies as potential antiviral therapeutics
- PMID: 40201976
- PMCID: PMC11988260
- DOI: 10.1080/19420862.2025.2486390
Nanoscale warriors against viral invaders: a comprehensive review of Nanobodies as potential antiviral therapeutics
Abstract
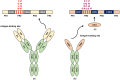
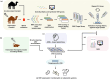
Viral infections remain a significant global health threat, with emerging and reemerging viruses causing epidemics and pandemics. Despite advancements in antiviral therapies, the development of effective treatments is often hindered by challenges, such as viral resistance and the emergence of new strains. In this context, the development of novel therapeutic modalities is essential to combat notorious viruses. While traditional monoclonal antibodies are widely used for the treatment of several diseases, nanobodies derived from heavy chain-only antibodies have emerged as promising "nanoscale warriors" against viral infections. Nanobodies possess unique structural properties that enhance their ability to recognize diverse epitopes. Their small size also imparts properties, such as improved bioavailability, solubility, stability, and proteolytic resistance, making them an ideal class of therapeutics for viral infections. In this review, we discuss the role of nanobodies as antivirals against various viruses. Techniques used for developing nanobodies, delivery strategies are covered, and the challenges and opportunities associated with their use as antiviral therapies are discussed. We also offer insights into the future of nanobody-based antiviral research to support the development of new strategies for managing viral infections.
Keywords: Antiviral; Single-domain antibodies; camelid antibodies; intranasal delivery; nanobodies; phage display; viral infections; virus.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Hoffman M, Chigbu DI, Crumley BL, Sharma R, Pustylnikov S, Crilley T, Ginwala R, Loonawat R, Joseph J, Sales D, et al. Human acute and chronic viruses: host-pathogen interactions and therapeutics [Internet]. In: Jain P, Ndhlovu L, editors. Advanced concepts in human immunology: prospects for disease control. Cham: Springer International Publishing; 2020. [cited 2024 Oct 1]. p. 1–23. doi: 10.1007/978-3-030-33946-31. - DOI
-
- Verma V. Leveraging monoclonal antibodies as therapeutics to address antimicrobial resistance in bacteria. J App Biol Biotech. 2022;11:53–60. doi: 10.7324/JABB.2023.90087. - DOI
-
- Resa-Infante P, Erkizia I, Muñiz-Trabudua X, Linty F, Bentlage AEH, Perez-Zsolt D, Muñoz-Basagoiti J, Raïch-Regué D, Izquierdo-Useros N, Rispens T, et al. Preclinical development of humanized monoclonal antibodies against CD169 as a broad antiviral therapeutic strategy. Biomed Pharmacother. 2024;175:116726. doi: 10.1016/j.biopha.2024.116726. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
