Noninvasive Temporal Interference Stimulation of the Subthalamic Nucleus in Parkinson's Disease Reduces Beta Activity
- PMID: 40202094
- PMCID: PMC12160966
- DOI: 10.1002/mds.30134
Noninvasive Temporal Interference Stimulation of the Subthalamic Nucleus in Parkinson's Disease Reduces Beta Activity
Abstract
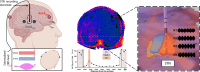
Background: Temporal interference stimulation (TIS) is a novel noninvasive electrical stimulation technique to focally modulate deep brain regions; a minimum of two high-frequency signals (f1 and f2 > 1 kHz) interfere to create an envelope-modulated signal at a deep brain target with the frequency of modulation equal to the difference frequency: Δf = |f2 - f1|.
Objective: The goals of this study were to verify the capability of TIS to modulate the subthalamic nucleus (STN) with Δf and to compare the effect of TIS and conventional deep brain stimulation (DBS) on the STN beta oscillations in patients with Parkinson's disease (PD).
Methods: DBS leads remained externalized after implantation, allowing local field potentials (LFPs) recordings in eight patients with PD. TIS was performed initially by two pairs (f1 = 9.00 kHz; f2 = 9.13 kHz, 4 mA peak-peak per pair maximum) of scalp electrodes placed in temporoparietal regions to focus the envelope signal maximum (Δf = 130 Hz) at the motor part of the STN target.
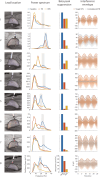
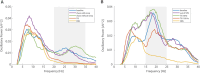
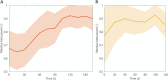
Results: The comparison between the baseline LFPs and recordings after TIS and conventional DBS sessions showed substantial suppression of high beta power peak after both types of stimulation in all patients.
Conclusions: TIS has the potential to effectively modulate the STN and reduce the beta oscillatory activity in a completely noninvasive manner, as is traditionally possible only with intracranial DBS. Future studies should confirm the clinical effectiveness of TIS and determine whether TIS could be used to identify optimal DBS candidates and individualize DBS targets. © 2025 The Author(s). Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Keywords: Parkinson's disease; beta power; deep brain stimulation; local field potentials; subthalamic nucleus; temporal interference stimulation.
© 2025 The Author(s). Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Figures
References
-
- Hill AV, Katz B, Solandt D. Nerve excitation by alternating current. Proc R Soc Lond B Biol Sci 1936;121(821):74–133. 10.1098/rspb.1936.0053 - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

