Repeated Exposure to Sevoflurane in Neonatal Mice Induces Cognitive and Synaptic Impairments in a TTLL6-Mediated Tubulin Polyglutamylation Manner
- PMID: 40202105
- PMCID: PMC11979716
- DOI: 10.1111/cns.70376
Repeated Exposure to Sevoflurane in Neonatal Mice Induces Cognitive and Synaptic Impairments in a TTLL6-Mediated Tubulin Polyglutamylation Manner
Abstract
Aims: Repeated sevoflurane exposure during the neonatal stage may induce Tau phosphorylation, dendritic spine loss, and neurocognitive impairment in the developing brain. Tubulin tyrosine ligase like-6 (TTLL6), which aggregates in dendrites due to Tau missorting, regulates microtubule stability via α-tubulin polyglutamylation. Meanwhile, Spastin modulates dendritic spine formation by severing microtubules. We hypothesize that repeated sevoflurane treatment impairs dendritic spine remodeling in neonatal mice by enhancing TTLL6-mediated tubulin polyglutamylation and increasing Spastin expression, leading to cognitive dysfunction in their pre-adolescent stage.
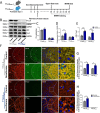
Methods: Six-day-old wild type (WT), TTLL6 brain conditional knockout (TTLL6CKO), TTLL6-flox (TTLL6CON) and Tau-knockout mice were treated with 3% sevoflurane for 2 h daily on postnatal days (P) 6, 8, and 10. Levels of Tau, phosphorylated Tau (pTau), TTLL6, polyglutamylated tubulin, ATP, Spastin, PSD95, Tau-TTLL6 interaction, Tau-TTLL6 missorting, dendritic spine remodeling, and behavioral alterations were compared across these groups.
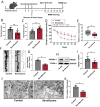
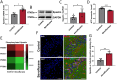
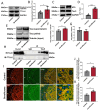
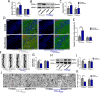
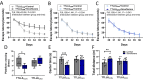
Results: Repeated sevoflurane exposure during brain development in neonatal mice could reduce dendritic spine density, synapse number, PSD95, and ATP levels, while increasing pTau, polyglutamylated tubulin, Tau-TTLL6 missorting from axons to the somatodendritic compartment, and Spastin levels, leading to cognitive impairment later in their pre-adolescent stage (P30). However, these changes were ameliorated in the TTLL6CKO mice.
Conclusions: Repeated neonatal sevoflurane exposure results in synaptic impairment through TTLL6-mediated tubulin polyglutamylation and increased Spastin expression, causing pre-adolescent cognitive dysfunction in mice. This process is initiated by Tau phosphorylation and missorting from axons to somatodendritic compartments.
Keywords: cognitive impairment; neonatal mice; sevoflurane; synaptic plasticity; tubulin polyglutamylation.
© 2025 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
Transparency statement: The design, data collection, analysis, and interpretation study adhere to rigorous scientific standards. All relevant data and methodologies have been clearly described in this manuscript, and any reproducible data are available upon request. There has been no selective reporting, and all hypotheses were based on a prespecified analysis plan.
The authors declare no conflicts of interest.
Figures
Similar articles
-
Tau missorting and spastin-induced microtubule disruption in neurodegeneration: Alzheimer Disease and Hereditary Spastic Paraplegia.Mol Neurodegener. 2015 Dec 21;10:68. doi: 10.1186/s13024-015-0064-1. Mol Neurodegener. 2015. PMID: 26691836 Free PMC article. Review.
-
Amyloid-β oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin.EMBO J. 2013 Nov 13;32(22):2920-37. doi: 10.1038/emboj.2013.207. Epub 2013 Sep 24. EMBO J. 2013. PMID: 24065130 Free PMC article.
-
Enriched environment mitigates cognitive impairment in pre-adolescent mice following repeated neonatal sevoflurane exposure by reducing TTBK1 expression and Tau phosphorylation.Neuropharmacology. 2025 May 1;268:110327. doi: 10.1016/j.neuropharm.2025.110327. Epub 2025 Jan 30. Neuropharmacology. 2025. PMID: 39892471
-
Anesthetic Sevoflurane Induces Enlargement of Dendritic Spine Heads in Mouse Neurons via Tau-Dependent Mechanisms.Anesth Analg. 2025 Mar 1;140(3):697-709. doi: 10.1213/ANE.0000000000006941. Epub 2024 Mar 20. Anesth Analg. 2025. PMID: 38507523
-
Role of the GABAA receptors in the long-term cognitive impairments caused by neonatal sevoflurane exposure.Rev Neurosci. 2019 Nov 26;30(8):869-879. doi: 10.1515/revneuro-2019-0003. Rev Neurosci. 2019. PMID: 31145696 Review.
References
-
- Wong‐Kee‐You A. M. B., Loveridge‐Easther C., Mueller C., Simon N., and Good W. V., “The Impact of Early Exposure to General Anesthesia on Visual and Neurocognitive Development,” Survey of Ophthalmology 68, no. 3 (2023): 539–555. - PubMed
-
- Yu Y., Yang M., Zhuang X., et al., “Neurotoxic 18‐kDa Apolipoprotein E Fragment Production Contributes to Anesthetic Sevoflurane‐Induced Tau Phosphorylation and Neuroinflammation In Vitro,” Human & Experimental Toxicology 41 (2022): 9603271221102519. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

