Optimized Mouse Model of Sepsis-Associated Encephalopathy: A Rational Standard Based on Modified SHIRPA Score and Neurobehaviors in Mice
- PMID: 40202114
- PMCID: PMC11979712
- DOI: 10.1111/cns.70365
Optimized Mouse Model of Sepsis-Associated Encephalopathy: A Rational Standard Based on Modified SHIRPA Score and Neurobehaviors in Mice
Abstract
Background: Sepsis-associated encephalopathy (SAE), a severe neurological disorder, is marked by widespread brain dysfunction. At present, there is no universally accepted criterion for diagnosing SAE in animal models. This study proposes a standardized evaluation method for SAE in mice, addressing inconsistencies in current research.
Method: Using a cecal ligation and puncture (CLP) model to induce sepsis, we assessed the physiological status of mice with a modified SHIRPA score to differentiate SAE from non-SAE, validating our findings through various behavioral tests and evaluations of neuroinflammation and neuronal damage.
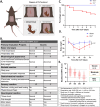
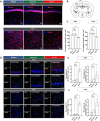
Results: Our findings revealed that the conventional mild-moderate-severe categorization of SHIRPA was insufficient for distinguishing between SAE and non-SAE. To enhance differentiation, we classified mice based on the median modified SHIRPA score, validating this approach through behavioral tests including the Y-maze, three-chamber social test, and open field test. This method effectively identified neurological impairments in septic mice. Further validation involved assessing neuronal damage, neuroinflammation, the Morris water maze, and long-term potentiation (LTP) in the hippocampal CA1 region. Results indicated that mice in the up-Median group exhibited greater neuroinflammation, neuronal injury, and cognitive deficits compared to the down-Median group.
Conclusions: This study establishes a reliable evaluation method for SAE in murine models, facilitating improved differentiation between SAE and non-SAE. Such advancements will enhance our understanding of the pathogenesis of SAE and guide more effective treatment strategies.
Keywords: behavioral tests; cognitive deficits; long‐term potentiation; modified SHIRPA score; sepsis‐associated encephalopathy.
© 2025 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
USP8 ameliorates cognitive and motor impairments via microglial inhibition in a mouse model of sepsis-associated encephalopathy.Brain Res. 2019 Sep 15;1719:40-48. doi: 10.1016/j.brainres.2019.05.009. Epub 2019 May 7. Brain Res. 2019. PMID: 31075263
-
Genetic differences in hippocampus of mice susceptible to sepsis-associated encephalopathy.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024 Nov 28;49(11):1777-1789. doi: 10.11817/j.issn.1672-7347.2024.240045. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 40177761 Free PMC article. Chinese, English.
-
Mesencephalic Astrocyte-Derived Neurotrophic Factor (MANF) Mitigates Neuroinflammation and Cognitive Impairment by Modulating Glial Activation in Sepsis-Associated Encephalopathy.Neurochem Res. 2024 Nov 29;50(1):39. doi: 10.1007/s11064-024-04296-5. Neurochem Res. 2024. PMID: 39612058
-
The Biological Changes of Synaptic Plasticity in the Pathological Process of Sepsis-associated Encephalopathy.Curr Neuropharmacol. 2025;23(4):359-374. doi: 10.2174/1570159X23666241028105746. Curr Neuropharmacol. 2025. PMID: 39473252 Free PMC article. Review.
-
Convergence of sepsis-associated encephalopathy pathogenesis onto microglia.J Transl Med. 2025 Jun 3;23(1):622. doi: 10.1186/s12967-025-06635-8. J Transl Med. 2025. PMID: 40462103 Free PMC article. Review.
References
-
- Kingsley S. M. and Bhat B. V., “Differential Paradigms in Animal Models of Sepsis,” Current Infectious Disease Reports 18, no. 9 (2016): 26. - PubMed
-
- Buras J. A., Holzmann B., and Sitkovsky M., “Animal Models of Sepsis: Setting the Stage,” Nature Reviews. Drug Discovery 4, no. 10 (2005): 854–865. - PubMed
-
- Dejager L., Pinheiro I., Dejonckheere E., and Libert C., “Cecal Ligation and Puncture: The Gold Standard Model for Polymicrobial Sepsis?,” Trends in Microbiology 19, no. 4 (2011): 198–208. - PubMed
MeSH terms
Grants and funding
- 82071311/National Natural Science Foundation of China
- 82072788/National Natural Science Foundation of China
- 2018SHZDZX01/Shanghai Municipal Science and Technology Major Projects
- 22ZR1413700/Shanghai Municipal Science and Technology Major Projects
- SHDC2020CR3021A/Shanghai Municipal Hospital Development Center
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

