A Porphyrin Nanomaterial for Photoimmunotherapy for Treatment of Melanoma
- PMID: 40202119
- PMCID: PMC12140306
- DOI: 10.1002/advs.202414592
A Porphyrin Nanomaterial for Photoimmunotherapy for Treatment of Melanoma
Abstract
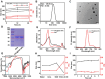
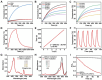
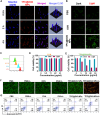
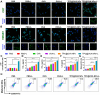
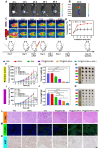
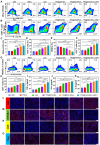
The incidence of melanoma, the third most common skin cancer, has been on the rise in recent years. In addition, it has a high mortality rate due to its high aggressiveness. Phototherapy, as a promising treatment method, can effectively kill tumor cells, but it is incapable of the treatment of tumor metastasis. Herein, a nanomaterial (TPC@OVA NPs) is developed for phototherapy in conjunction with immunotherapy against melanoma. TPC, as a derivative of porphyrin, is used as a photosensitizer with excellent biosafety and photostability. After assembly with ovalbumin (OVA), TPC@OVA NPs with vaccine properties is formed, which can not only ablate the primary tumor but also induce immunogenic cell death (ICD). In addition, DC cells can be stimulated to mature by exogenous OVA, enhancing the immune response against tumors by further activating T lymphocytes. Combined with immune checkpoint inhibitor aPD-1, the immune microenvironment is reshaped, and the increased activity of immunotherapy are validated. This work highlights the potential of combining phototherapy and immunotherapy against metastasis.
Keywords: combination therapy; immunogenic cell death; melanoma; phototherapy; tumor vaccine.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
NIR II light-driven nanomotor synergistically enhances immunogenic cell death through photothermal and chemodynamic therapy for melanoma immunotherapy.J Colloid Interface Sci. 2025 Sep 15;694:137688. doi: 10.1016/j.jcis.2025.137688. Epub 2025 Apr 23. J Colloid Interface Sci. 2025. PMID: 40300374
-
Design of a targeted dual drug delivery system for boosting the efficacy of photoimmunotherapy against melanoma proliferation and metastasis.J Adv Res. 2025 May;71:533-550. doi: 10.1016/j.jare.2024.05.017. Epub 2024 May 19. J Adv Res. 2025. PMID: 38768811 Free PMC article.
-
Multi-pathway oxidative stress amplification via controllably targeted nanomaterials for photoimmunotherapy of tumors.J Nanobiotechnology. 2025 Jan 22;23(1):33. doi: 10.1186/s12951-025-03116-4. J Nanobiotechnology. 2025. PMID: 39844145 Free PMC article.
-
Nanomaterial-Based Tumor Photothermal Immunotherapy.Int J Nanomedicine. 2020 Nov 19;15:9159-9180. doi: 10.2147/IJN.S249252. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33244232 Free PMC article. Review.
-
Nanotechnology-based photo-immunotherapy: a new hope for inhibition of melanoma growth and metastasis.J Drug Target. 2023 Jul;31(6):555-568. doi: 10.1080/1061186X.2023.2216402. Epub 2023 May 29. J Drug Target. 2023. PMID: 37216425 Review.
References
MeSH terms
Substances
Grants and funding
- 2020SCZ56/Jilin Province Health Research Talent Special Project
- 2021SCZ38/Jilin Province Health Research Talent Special Project
- 2022SCZ08/Jilin Province Health Research Talent Special Project
- 2020SCZ38/Jilin Province Health Research Talent Special Project
- JJKH20211143KJ/Science and Technology Research Project of the Education Department of Jilin Province
LinkOut - more resources
Full Text Sources
Medical
