Diagnostic Value of Serum Cytokeratin 18 for the Staging of Liver Inflammation and Fibrosis: A Meta-Analysis
- PMID: 40202219
- PMCID: PMC12019703
- DOI: 10.1002/jcla.70034
Diagnostic Value of Serum Cytokeratin 18 for the Staging of Liver Inflammation and Fibrosis: A Meta-Analysis
Abstract
Background and aims: Accurate assessment of liver inflammation and fibrosis is of vital importance in the clinical management of patients with liver diseases. Our aim is to conduct a meta-analysis to evaluate the diagnostic accuracy of serum cytokeratin 18 (CK18) for staging of liver inflammation and fibrosis against a liver biopsy in adults.
Methods: We systematically searched articles from eight electronic databases. Two authors independently selected included studies, extracted data, and assessed quality. In our meta-analysis, we used the random-effects meta-analysis model. Publication bias, sensitivity analysis, heterogeneity analysis, and post-test probability were used in this meta study.
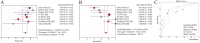
Results: A total of 20 studies with 2235 patients were initially found by the search strategies. The pooled sensitivity, specificity, and area under the curve (AUC) of the summary receiver operating characteristic curve were 0.56, 0.81, and 0.810 for significant fibrosis; 0.64, 0.76, and 0.785 for advanced fibrosis; 0.53, 0.76, and 0.830 for cirrhosis; and 0.68, 0.73, and 0.786 for significant inflammation, respectively. High heterogeneity was observed in our meta-analysis because of factors such as the proportion of males, total number, and antigens of CK-18.
Conclusion: Serum CK18 had moderate diagnostic value (AUC > 0.7) in different stages of liver fibrosis and significant inflammation, offering a complementary approach to other non-invasive indicators such as serological biomarkers and imaging techniques. Future research should focus on elucidating the role of CK18 in the occurrence and progression of hepatitis and liver fibrosis, particularly in liver diseases with diverse etiologies.
Keywords: cytokeratin 18; diagnostic; fibrosis; inflammation; liver biopsy.
© 2025 The Author(s). Journal of Clinical Laboratory Analysis published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- European Association for the Study of the Liver (EASL) , European Association for the Study of Diabetes (EASD) , and European Association for the Study of Obesity (EASO) , “EASL‐EASD‐EASO Clinical Practice Guidelines for the Management of Non‐Alcoholic Fatty Liver Disease,” Diabetologia 59, no. 6 (2016): 1121–1140, 10.1007/s00125-016-3902-y. - DOI - PubMed
-
- Chinese Society of Infectious Diseases, Chinese Medical Association and Chinese Society of Hepatology, Chinese Medical Association , “Guidelines for the Prevention and Treatment for Chronic Hepatitis B (Version 2019),” Zhonghua Gan Zang Bing Za Zhi 2020 (2020): 21–22.
-
- National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association, Fatty Liver Expert Committee, Chinese Medical Doctor Association , “Guidelines of Prevention and Treatment for Nonalcoholic Fatty Liver Disease: A 2018 Update,” Infectious Disease Information 31, no. 5 (2018): 393–402, 10.3760/cma.j.issn.1007-3418.2018.03.008. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

