Improved and Automated Detection of Papillary Muscle Infarction Using Joint Bright- and Black-Blood Late Gadolinium Enhancement MRI
- PMID: 40202270
- PMCID: PMC12335337
- DOI: 10.1002/jmri.29777
Improved and Automated Detection of Papillary Muscle Infarction Using Joint Bright- and Black-Blood Late Gadolinium Enhancement MRI
Abstract
Background: Papillary muscle infarction (PMI) has been linked to significantly increased mortality and is associated with ventricular arrhythmias and mitral regurgitation. Reference bright-blood late gadolinium enhancement (LGE) imaging provides poor scar-to-blood contrast, making PMI visualization challenging. Black-blood LGE imaging overcomes this limitation by improving the blood-scar contrast.
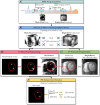
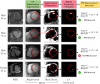
Purpose: To evaluate a recent co-registered bright- (papillary muscle localization) and black-blood (PMI visualization) sequence (Scar-specific imaging with Preserved myOcardial visualizaTion: SPOT) to improve PMI visualization compared to a reference standard phase-sensitive inversion recovery (PSIR) sequence, and to enable automated PMI detection (auto-PMI).
Study type: Retrospective.
Population: 198 patients with ischemic heart disease were divided into an optimization dataset (N = 127) and a testing dataset (N = 71).
Field strength/sequence: 2D SPOT and PSIR balanced steady-state free precession sequences at 1.5 T.
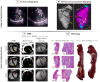
Assessment: Auto-PMI included: image acquisition, slice selection, endocardial segmentation, blood pool preprocessing, and PMI detection. Three radiologists (8, 5 and 2 years of MRI experience) assessed PMI in SPOT and PSIR images independently. A consensus reading regarding all assessments of both sequences was established. The number of patients with PMI in SPOT and PSIR acquisitions was compared. The diagnostic performances of visual (SPOT and PSIR) and auto-PMI (SPOT) detection were evaluated. Inter- and intra-observer reproducibility of the visual PMI detection was assessed.
Statistical tests: McNemar test, p-value < 0.05 was considered statistically significant.
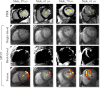
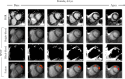
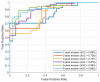
Results: In the testing dataset, significantly more patients with PMI were detected using SPOT compared to PSIR in each session (37 vs. 27, 36 vs. 29, 41 vs. 31, 42 vs. 25). Sensitivity ranges for visual PMI detection were significantly higher using SPOT (89%-100% vs. 61%-82%). SPOT vs. PSIR inter- and intra-observer reproducibility ranges were 77%-80% vs. 71%-77%, and 97% vs. 88%, respectively. Auto-PMI sensitivity was 87%.
Data conclusion: Co-registered bright- and black-blood SPOT imaging improved visual PMI detection and facilitated automated PMI assessment.
Evidence level: 3. Technical Efficacy: Stage 2.
Keywords: automated detection; late gadolinium enhancement; papillary muscle infarction; scar‐specific imaging with preserved myocardial visualization.
© 2025 The Author(s). Journal of Magnetic Resonance Imaging published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Figures
Similar articles
-
Influence of Reader Expertise on Myocardial Infarction Detection: A Comparative Study of Dark-Blood and Bright-Blood Late Gadolinium Enhancement MRI.Invest Radiol. 2025 Sep 1;60(9):561-568. doi: 10.1097/RLI.0000000000001161. Invest Radiol. 2025. PMID: 39983023 Free PMC article.
-
Comparison of fast and standard segmented techniques for detection of late gadolinium enhancement in acute myocardial infarction: a prospective clinical cardiovascular magnetic resonance trial.Quant Imaging Med Surg. 2025 Jun 6;15(6):5769-5780. doi: 10.21037/qims-24-2308. Epub 2025 Jun 3. Quant Imaging Med Surg. 2025. PMID: 40606345 Free PMC article.
-
3D whole-heart phase sensitive inversion recovery CMR for simultaneous black-blood late gadolinium enhancement and bright-blood coronary CMR angiography.J Cardiovasc Magn Reson. 2017 Nov 27;19(1):94. doi: 10.1186/s12968-017-0405-z. J Cardiovasc Magn Reson. 2017. PMID: 29178893 Free PMC article.
-
Contrast-enhanced ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: a systematic review and cost-effectiveness analysis.Health Technol Assess. 2013 Apr;17(16):1-243. doi: 10.3310/hta17160. Health Technol Assess. 2013. PMID: 23611316 Free PMC article.
-
Systematic review and modelling of the cost-effectiveness of cardiac magnetic resonance imaging compared with current existing testing pathways in ischaemic cardiomyopathy.Health Technol Assess. 2014 Sep;18(59):1-120. doi: 10.3310/hta18590. Health Technol Assess. 2014. PMID: 25265259 Free PMC article.
References
-
- Okayama S., Uemura S., Soeda T., et al., “Clinical Significance of Papillary Muscle Late Enhancement Detected via Cardiac Magnetic Resonance Imaging in Patients With Single Old Myocardial Infarction,” International Journal of Cardiology 146, no. 1 (2011): 73–79, 10.1016/j.ijcard.2010.04.037. - DOI - PubMed
-
- Bouma W., Willemsen H. M., Lexis C. P., et al., “Chronic Ischemic Mitral Regurgitation and Papillary Muscle Infarction Detected by Late Gadolinium‐Enhanced Cardiac Magnetic Resonance Imaging in Patients With ST‐Segment Elevation Myocardial Infarction,” Clinical Research in Cardiology 105, no. 12 (2016): 981–991, 10.1007/s00392-016-1006-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

