GCKR Polymorphisms Increase the Risks of Low Bone Mineral Density in Young and Non-Obese Patients With MASLD and Hyperuricemia
- PMID: 40202351
- PMCID: PMC12199583
- DOI: 10.1002/kjm2.70017
GCKR Polymorphisms Increase the Risks of Low Bone Mineral Density in Young and Non-Obese Patients With MASLD and Hyperuricemia
Abstract
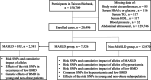
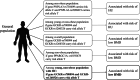
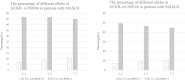
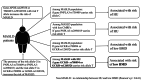
Metabolic-associated steatotic liver disease (MASLD) encompasses common comorbidities including low bone mineral density (BMD) and hyperuricemia (HU), yet relevant genetic analyses are limited. This study aimed to investigate the genetic effects of risk single nucleotide polymorphisms (SNPs) on the occurrence of low BMD in patients with MASLD and HU, particularly focusing on relatively young or non-obese populations. We conducted a cross-sectional study utilizing data from the Taiwan Biobank, screening a total of 150,709 participants who were prospectively enrolled over a period of 13 years. The risk SNPs for MASLD were identified. Genotype analyses of HU and its effects on the occurrence of low BMD in the general population were evaluated, with further analyses of common SNPs focusing on patients with MASLD, including subgroup analyses on relatively young and non-obese populations. A total of 20,496 participants were eligible for analysis, including 7526 patients with MASLD. Several risk SNPs for MASLD were identified. Furthermore, MASLD patients carrying the PNPLA3-rs738409 C_C, PNPLA3-rs2896019 T_T, GCKR-rs780094 T_T, and GCKR-rs1260326 T_T genotypes exhibited an increased risk of comorbidity with HU. Trend analysis revealed that the T alleles in GCKR-rs780094 and GCKR-rs1260326 were associated with the occurrence of low BMD in MASLD individuals comorbid with HU, particularly among relatively young or non-obese populations. In relatively young, non-obese patients with MASLD and HU, genetic effects significantly increase the risk of occurrence of low BMD. Given the presence of genetic effects in these ostensibly low-risk groups, heightened awareness and close follow-up are recommended.
Keywords: hyperuricemia; low bone mineral density; metabolic‐associated steatotic liver disease; single nucleotide polymorphisms.
© 2025 The Author(s). The Kaohsiung Journal of Medical Sciences published by John Wiley & Sons Australia, Ltd on behalf of Kaohsiung Medical University.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Clinical Utility of Genetic Variants in PNPLA3 and TM6SF2 to Predict Liver-Related Events in Metabolic Dysfunction-Associated Steatotic Liver Disease.Liver Int. 2025 Apr;45(4):e16124. doi: 10.1111/liv.16124. Epub 2024 Oct 7. Liver Int. 2025. PMID: 39373247
-
Polymorphism's MBOAT7 as Risk and MTARC1 as Protection for Liver Fibrosis in MASLD.Int J Mol Sci. 2025 Jul 3;26(13):6406. doi: 10.3390/ijms26136406. Int J Mol Sci. 2025. PMID: 40650184 Free PMC article.
-
The Distribution and Survival Association of Genetic Polymorphisms in Thai Patients with Hepatocellular Carcinoma According to Underlying Liver Disease.Genes (Basel). 2025 Jul 9;16(7):808. doi: 10.3390/genes16070808. Genes (Basel). 2025. PMID: 40725464 Free PMC article.
-
PNPLA3 I148M Interacts With Environmental Triggers to Cause Human Disease.Liver Int. 2025 Mar;45(3):e16106. doi: 10.1111/liv.16106. Epub 2024 Nov 19. Liver Int. 2025. PMID: 39559944 Free PMC article. Review.
-
Meta-Analysis: Effects of Steatotic Liver Disease-Associated Genetic Risk Alleles on Longitudinal Outcomes.Aliment Pharmacol Ther. 2025 Aug;62(3):244-276. doi: 10.1111/apt.70256. Epub 2025 Jun 28. Aliment Pharmacol Ther. 2025. PMID: 40580198 Free PMC article.
References
-
- Tao J., Li H., Wang H., Tan J., and Yang X., “Metabolic Dysfunction‐Associated Fatty Liver Disease and Osteoporosis: The Mechanisms and Roles of Adiposity,” Osteoporosis International 35 (2024): 1–10. - PubMed
-
- Dehlin M., Jacobsson L., and Roddy E., “Global Epidemiology of Gout: Prevalence, Incidence, Treatment Patterns and Risk Factors,” Nature Reviews Rheumatology 16 (2020): 380–390. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

