Plasticity of mitotic cyclins in promoting the G2-M transition
- PMID: 40202486
- PMCID: PMC11980681
- DOI: 10.1083/jcb.202409219
Plasticity of mitotic cyclins in promoting the G2-M transition
Abstract
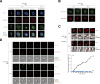
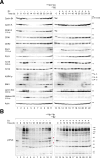
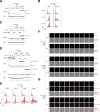
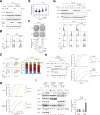
Cyclins and cyclin-dependent kinases (CDKs) orchestrate key events in the cell cycle. However, the uniqueness of individual mitotic cyclins has been a long-standing puzzle. By rapidly removing cyclins in G2 human cells, we found that deficiency of B-type cyclins attenuates mitotic onset and uncouples the G2-M kinase network from mitosis, resulting in sustained activation of PLK1 and cyclin A-CDK1. This culminates in mitotic slippage without completing nuclear envelope breakdown. Remarkably, elevating cyclin A several-fold above its endogenous level is adequate to restore mitosis, allowing cells to survive without B-type cyclins. In contrast, cyclin A is rate-limiting but not essential for G2-M due to compensation by endogenous cyclin B1-CDK2, a non-canonical pair. These findings challenge the traditional indispensable roles of different cyclins and highlight their plasticity. Due to the high malleability of the A- and B-type cyclins, cancer cells may be able to place different weights on different cyclins, while maintaining sufficient CDK activities for successful mitosis.
© 2025 Crncec et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

