Mutated sigma-1R disrupts cell homeostasis in dHMN patient cells
- PMID: 40202561
- PMCID: PMC11981993
- DOI: 10.1007/s00018-025-05676-y
Mutated sigma-1R disrupts cell homeostasis in dHMN patient cells
Abstract
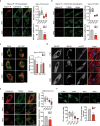
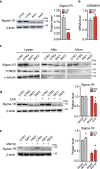
Hereditary-Motor-Neuropathies (dHMNs) are clinically and genetically heterogeneous neurological disorders characterized by degeneration of peripheral motoneurons. We previously identified two sigma-1 receptor (Sigma-1R) variants (p.E138Q; p.E150K) in dHMN Italian patients that behave as "loss-of-function" mutations in neuroblastoma cell lines. Here, we characterize the functional effects of Sigma-1R mutation in primary fibroblasts from homozygous patients bearing the E150K mutation, and matched controls, by performing biochemical, gene expression, immunofluorescence and Ca2+ imaging analysis. Our results show that Sigma-1R expression and distribution is significantly altered in patient fibroblasts. Moreover, patient cells present a general derangement of cell homeostasis as revealed by impairment of global Ca2+ dynamics, disorganization of the ER-mitochondria tethers, enhancement of the autophago-lysosomal pathway and blunting of mitochondrial aerobic metabolism compared to controls. These findings highlight the crucial role of Sigma-1R in the maintenance of cell and protein homeostasis, inter-organelle communication and intracellular Ca2+ signalling, supporting the notion that Sigma-1R is protective for motor neuron activity and its down-regulation and/or loss-of-function, as in the case of the E150K mutation, might play the key role in the neuronal degeneration in dHMN patients.
Keywords: Ca2+ signalling; Cellular proteostasis; ER-mitochondria contacts; Hereditary neuropathies; Sigma-1R.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors have no relevant financial or non-financial interests to disclose. Ethics approval and consent to participate: This study was performed in line with the principles of the Declaration of Helsinki. The skin primary fibroblasts were achieved from selected patients after obtaining written consent to participate to the research study, according to the procedures of the Centre for Neuromuscular and Neurological Rare Diseases, S. Camillo Forlanini Hospital, Rome, Italy. Consent to publish: Not applicable.
Figures



Similar articles
-
Loss-of-function mutations in the SIGMAR1 gene cause distal hereditary motor neuropathy by impairing ER-mitochondria tethering and Ca2+ signalling.Hum Mol Genet. 2016 Sep 1;25(17):3741-3753. doi: 10.1093/hmg/ddw220. Epub 2016 Jul 8. Hum Mol Genet. 2016. PMID: 27402882
-
Sigma-1 Receptor Activation Suppresses Microglia M1 Polarization via Regulating Endoplasmic Reticulum-Mitochondria Contact and Mitochondrial Functions in Stress-Induced Hypertension Rats.Mol Neurobiol. 2021 Dec;58(12):6625-6646. doi: 10.1007/s12035-021-02488-6. Epub 2021 Oct 2. Mol Neurobiol. 2021. PMID: 34601668
-
N, N-Dimethyltryptamine, a natural hallucinogen, ameliorates Alzheimer's disease by restoring neuronal Sigma-1 receptor-mediated endoplasmic reticulum-mitochondria crosstalk.Alzheimers Res Ther. 2024 May 1;16(1):95. doi: 10.1186/s13195-024-01462-3. Alzheimers Res Ther. 2024. PMID: 38693554 Free PMC article.
-
Knocking Out Sigma-1 Receptors Reveals Diverse Health Problems.Cell Mol Neurobiol. 2022 Apr;42(3):597-620. doi: 10.1007/s10571-020-00983-3. Epub 2020 Oct 23. Cell Mol Neurobiol. 2022. PMID: 33095392 Free PMC article. Review.
-
Sigma-1 Receptor in Motoneuron Disease.Adv Exp Med Biol. 2017;964:235-254. doi: 10.1007/978-3-319-50174-1_16. Adv Exp Med Biol. 2017. PMID: 28315275 Review.
References
-
- Hayashi T, Su TP (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131:596–610. 10.1016/j.cell.2007.08.036 - PubMed
-
- Renaudo A, Watry V, Chassot AA et al (2004) Inhibition of tumor cell proliferation by σ ligands is associated with K+ channel inhibition and p27kip1 accumulation. J Pharmacol Exp Ther 311:1105–1114. 10.1124/JPET.104.072413 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

