Short-lived neutralizing activity against SARS-CoV-2 in newborns of immunized mothers
- PMID: 40202786
- PMCID: PMC11980968
- DOI: 10.1111/pai.70084
Short-lived neutralizing activity against SARS-CoV-2 in newborns of immunized mothers
Abstract
Background: Newborns under 6 months of age are at high risk of hospitalization for acute respiratory failure following SARS-CoV-2 infection. Herein, we analyzed neonatal protection against SARS-CoV-2 passively acquired after mother vaccination and/or infection (hybrid immunity).
Methods: We enrolled seventy-eight newborns of immunized mothers against SARS-CoV-2 before or during pregnancy, through vaccination and/or infection. Infants were stratified based on the anamnestic lack (SARS-CoV-2 Vaccinated - SV)/presence (SARS-CoV-2 Infected and Vaccinated - SIV) of COVID-19 maternal infection. SARS-CoV-2-specific Neutralizing Activity (NA) in plasma was assessed by virus neutralization assay (vNTA) against the SARS-CoV-2 Omicron strain at delivery (T0), 3 and 6 months after birth (T3 and T6). Cytokine and chemokine profiles in newborns were also analyzed.
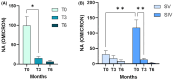
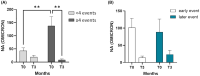
Results: At birth, significantly lower NA was observed in infants of SV compared to that of SIV mothers; NA declined equally in both groups 3 months after delivery. The presence of at least 4 immunizing events in the mother significantly enhances the NA against SARS-CoV-2 in newborns, regardless of the type of immunization (vaccination or hybrid immunity) and of the timing of the last maternal immunization. Finally, cytokines and chemokines plasma levels were high at birth in all newborns, followed by a decline over the subsequent month.
Conclusion: Our findings suggest that, independently of a previous SARS-CoV-2 infection or vaccination, it is reasonable to upgrade the recommendation of a booster dose during pregnancy to a "strongly recommended" status, with a view to conferring protection to newborns in the first months after delivery.
Keywords: SARS‐CoV‐2; hybrid immunity; neutralizing activity; newborns; passive immunity.
© 2025 The Author(s). Pediatric Allergy and Immunology published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


Similar articles
-
Timing of maternal vaccination against COVID-19 for effective protection of neonates: cohort study.Front Immunol. 2024 Jul 8;15:1359209. doi: 10.3389/fimmu.2024.1359209. eCollection 2024. Front Immunol. 2024. PMID: 39040104 Free PMC article.
-
Maternal Immunization Against SARS-CoV-2 and Infant Immunity Persistence in a Brazilian Cohort.Pediatr Infect Dis J. 2025 Apr 9;44(7):e242-e246. doi: 10.1097/INF.0000000000004817. Pediatr Infect Dis J. 2025. PMID: 40238645
-
Diversified humoral immunity and impacts of booster vaccines: SARS-CoV-2 antibody profile and Omicron BA.2 neutralization before and after first or second boosters.Microbiol Spectr. 2024 Oct 3;12(10):e0060524. doi: 10.1128/spectrum.00605-24. Epub 2024 Aug 20. Microbiol Spectr. 2024. PMID: 39162540 Free PMC article.
-
SARS-CoV-2 infection vs. vaccination during pregnancy: the placenta leads the way.Curr Opin Infect Dis. 2024 Oct 1;37(5):402-406. doi: 10.1097/QCO.0000000000001050. Epub 2024 Jul 31. Curr Opin Infect Dis. 2024. PMID: 39105669 Review.
-
Impact of Maternal Exposure to SARS-CoV-2 on Immunological Components of Breast Milk.Int J Mol Sci. 2025 Mar 13;26(6):2600. doi: 10.3390/ijms26062600. Int J Mol Sci. 2025. PMID: 40141241 Free PMC article. Review.
Cited by
-
Pregnancy reduces COVID-19 vaccine immunity against novel variants.NPJ Vaccines. 2025 Aug 13;10(1):191. doi: 10.1038/s41541-025-01236-4. NPJ Vaccines. 2025. PMID: 40804265 Free PMC article.
References
-
- Halasa NB, Olson SM, Staat MA, et al. Maternal vaccination and risk of hospitalization for Covid‐19 among infants. Obstet Gynecol Surv. 2023;78:3‐5.
-
- Barros FC, Gunier RB, Rego A, et al. Maternal vaccination against COVID‐19 and neonatal outcomes during omicron: INTERCOVID‐2022 study. Am J Obstet Gynecol. 2024;231:460.e1‐460.e17. - PubMed
-
- Abu Raya B, Edwards KM, Scheifele DW, Halperin SA. Pertussis and influenza immunisation during pregnancy: a landscape review. Lancet Infect Dis. 2017;17:e209‐e222. - PubMed
-
- Kampmann B, Madhi SA, Munjal I, et al. Bivalent Prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388:1451‐1464. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

