Reduced IgG1 Antibody Left-Twisted Antiparallel β-Sheet Structure Stability Occurs under Metal-Catalyzed Oxidation Conditions in the Presence of Polysorbates
- PMID: 40202920
- PMCID: PMC12118537
- DOI: 10.1021/acs.molpharmaceut.5c00034
Reduced IgG1 Antibody Left-Twisted Antiparallel β-Sheet Structure Stability Occurs under Metal-Catalyzed Oxidation Conditions in the Presence of Polysorbates
Abstract
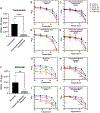
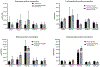
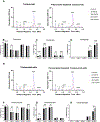
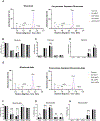
Polysorbates are common surfactants in monoclonal antibody (mAb) drug products. While polysorbates assist in stabilizing and refolding proteins, oxidative stress conditions can reduce protein stability wherein polysorbate binds to the oxidized and unfolded protein. We investigated the effects of polysorbates on the higher-order structural stability of mAbs under oxidative conditions that may occur during manufacturing, storage, and use. Secondary and tertiary structures of trastuzumab and rituximab products were investigated under two oxidative conditions: metal-catalyzed oxidation (MCO; CuSO4 and ascorbic acid) and 2,2'-azobis (2-aminidinopropane) dihydrochloride (AAPH) using either polysorbate-containing formulations or after polysorbate depletion. Higher-order structures were predicted from the collected circular dichroism spectra with an algorithm optimized for β-sheet structural predictions. Secondary structure analyses using circular dichroism at increasing temperatures demonstrated that MCO and AAPH triggered differing β-sheet structure degradation patterns. Rituximab products were more sensitive to MCO compared with trastuzumab products as shown by left-twisted antiparallel β-sheet structure loss and increase in unstructured elements at lower temperatures. AAPH-exposed drugs tended to have distinct unfolding states compared with the MCO-treated drugs as shown by the increase in parallel β-sheet structures for AAPH and decreased parallel β-sheet structures with MCO. Polysorbate depletion transiently improved the stability of MCO-treated material as shown by delayed circular dichroism (CD) signal degradation at 202 nm and improved peak area of the antibody monomer by nonreducing capillary electrophoresis sodium dodecyl sulfate (nrCE-SDS) and peak intensity of intact antibody in matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) analysis. The improved stability of detergent-depleted material is traced to improved stability in the predicted left-twisted β-sheet structural elements. Our data further highlights the need for formulation studies that consider the impact of polysorbate binding and/or degradation for specific drug products under stress conditions such as metal-catalyzed oxidation.
Keywords: higher-order structure; monoclonal antibody; oxidation; polysorbate; stability.
Figures





References
-
- Kerwin BA Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. Journal of pharmaceutical sciences 2008, 97 8, 2924–2935. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

