Field validation and application of the luminex triplex HIV assay to estimate HIV prevalence and HIV-1 incidence in Nigeria
- PMID: 40202973
- PMCID: PMC11981228
- DOI: 10.1371/journal.pgph.0003455
Field validation and application of the luminex triplex HIV assay to estimate HIV prevalence and HIV-1 incidence in Nigeria
Abstract
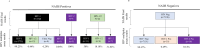
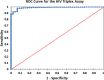
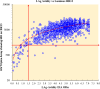
HIV cross-sectional surveys require multi-layered testing with several tests to estimate HIV prevalence and HIV-1 incidence. We evaluated the performance and accuracy of the newly developed HIV Triplex assay to diagnose HIV-1 and HIV-2 and detect HIV-1 recent infections using plasma samples from the 2018 Nigeria AIDS Indicator and Impact Survey (NAIIS). Plasma samples from consenting HIV-positive (n=2,773) and a subset of HIV-negative samples (n=7,196), as determined by the national rapid testing algorithm, followed by Bio-Rad Geenius HIV-1/2 Supplemental Assay and Western Blot, aged 18 months - 64 years, were tested using the Luminex-based HIV Triplex assay. The assay classified specimens as HIV-1 positive, HIV-2 positive, dual (HIV-1 & 2) infections, or HIV-seronegative. All HIV-1 and dual infections were further classified as either HIV-1 recent (<6 months) or long-term (LT) based on mean fluorescent intensities and compared with the LAg-Avidity EIA as the reference. Multiplex results were analyzed and compared with the final NAIIS survey data for unweighted HIV prevalence and HIV-1 incidence. The diagnostic sensitivity and specificity of the HIV Triplex assay was 99.71% and 99.37%, respectively, with a kappa of 0.987 when compared to NAIIS survey results. Percent agreement between the HIV Triplex assay and the LAg-Avidity EIA for recent and LT classification was 98.86% with a kappa of 0.80 [CI: 0.71-0.89] and a Spearman-ranked correlation (ρ) of 0.689. A small number (n=45; 0.63%) of the subset of negatives tested were classified by the multiplex assay as either HIV-1 positive (n=35) or HIV-2 positive (n=10). Nevertheless, the HIV Triplex assay agreed with NAIIS HIV-negative survey results (99.37%). Using these results as they were, unweighted estimates of HIV prevalence for both HIV Triplex assay and NAIIS test results were similar (1.62% [95% CI: 1.56-1.68] and 1.60% [95% CI: 1.54-1.66], respectively) with overlapping confidence. After adjusting for viral load and anti-retroviral therapy, HIV-1 unweighted incidence for ages ≥15 years, using HIV Triplex assay data, was 0.70 per 1,000 [95% CI: 0.40-0.90]. This is similar to the unweighted incidence using the LAg-based RITA (recent infection testing algorithm) of 0.80 per 1,000 [95% CI: 0.60-1.10]. The HIV Triplex assay combines several assays in one, providing highly accurate results for estimating HIV prevalence and HIV-1 incidence in surveys. This assay has the potential to simplify cross-sectional surveys making them less expensive, easier, and quicker.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interest exist.
Figures
References
-
- UNAIDS. Global HIV Statistics. 2021.
-
- Centers for Disease Control and Prevention. Prevention of Pneumocystis pneumonia. MMWR Morb Mortal Wkly Rep. 1981;30(21):250–2. - PubMed
-
- Prevention CfDCa. Compendium of evidence-based interventions and best practices for HIV prevention 2021 July 27, 2021.
LinkOut - more resources
Full Text Sources
